Metallacrown Project
Background
Metallacrowns are inorganic analogues of crown ethers. A typical crown ether is a cyclic oligomer with a -C-C-O- repeat unit forming the macrocyclic ring. In a metallacrown, a transition metal ion substitutes for one of the carbons in the ethylene bridge of the ether ring and a nitrogen substitues for the other. The repeat unit in a metallacrown, thus, becomes -M-N-O-. As shown in fig 1, metallacrowns have similar backbone structure and ring sizes as their organic counterparts. To date, three sizes have been isolated:
9-MC-3, 12-MC-4, 15-MC-5, where MC stands for Metallacrown as an analogy to the naming system developed for crown ethers.
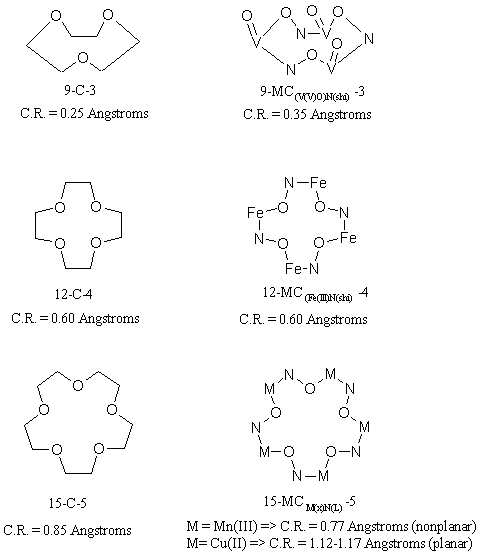
Figure 1. The Crown Analogy
The first metallacrowns was isolated while exploring the biological chemistry of the sallicylhydroximate complexes of vanadium. This 1989 achievement was soon followed by the isolation of many complexes involving many metals, hydroximate ligands and ring sizes. Transition metals were used as ring metals as well as cavity metals, and lanthanides and actinides, now, are encapsulated by the 15-MC-5 structural motif. In the design of the metallacrown, many considerations were taken. The ring size is greatly dependent on the size of ligand used and its size. The oxidation state of the ring metal and the number of negatively charged groups on the ligand play a role in limiting the number of ligands or transition metals used in synthesis. The stability of a metallacrown in different solvent systems is also a consideration in the design.
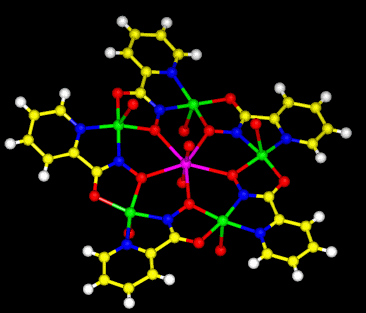
Figure 2. Crown ethers are classic ligands (composed of a cyclic polyether structure) that selectively sequester monovalent alkali metals and divalent alkali earths. The metallacrown shown here is an inorganic analogue of the crown ethers. The 15-metallacrown-5 contains Cu(II)-N-O repeats in order to form a 15-membered ring that can efficiently complex uranyl ion.
Nomenclature
Nomenclature designed for metallacrowns stem from the naming system of the organic crown ethers.The general naming formula is:CMn+(A-)n[X-MCM(X)T(L)-Y] where CMn+ stand for cavity metal with its charge shown on the upper right, and A- is the anion part of the cation/anion pair in the cavity of the crown. X indicates the size of the backbone ring, and Y is the number of oxygen atoms in the ring. MC stands for metallacrown, and L is the organic hydroxamic ligand used in synthesis, which is usually abbreviated into the conventional two or three letter system (for example:Picoline hydroxamic acid is abbreviated as Pic, glycine hydroxamic acid is abbreviated as Gly...). T is a symbol for the heterogeneous atom which is, so far nitrogen. M(X) is the ring metal with its oxidation number shown in brackets. For example, a planar metallacrown that resembles the 15-C-5 structural motif synthesized with Leucine Hydroxamic acid as the ligand, copper as a ring metal, and Europium as its cavity metal is represented as Eu(III)(NO3-)3[15-MC-Cu(II)N(Leu)-5] .
Project Goals
- Synthesis of large rings such as 18-MC-6.
- Incorporation of new ring metals, new cavity metals and new ligands in the synthesis of metallacrowns.
- Exploring the tremendous amount of applications proposed for metallacrowns as discussed below.
- Examination of the stability of metallacrowns and their dynamics in solution.
- Synthesis of metallacrowns with ligands having potentially active side groups which will open doors
to new metallacrown reactions and allow for the appendage of chemically important side chains.
Applications of such a project are countless. - Developement of functionalized metallacrowns for use in material applications.
- Designing rings of special characteristics relevant to specific proposed applications.
Applications and Current Work
Metallacrowns of all sizes can selectively encapsulate metals and anions in their cavities and are, therefore a new class of molecular recognition agents. Those recognize not just a cation or an anion, but can also selectively, bind cation/anion pairs. In fact, it has been shown that the size of the metallacrown, the solvent environment and salt bridges connecting the ring metals with the cavity metal can effectively screen for the metal cations. for example, NMR studies of 15-MC-5's selectivity demonstrated the preference of those rings for captivating uranil ions over others, and are therefore, good candidates for applications in the fields of identification and removal of chemical deposits of the cold war. The special design of metallacrowns with specific targetting abilities is an important goal in our lab. This involves varying the cavity size, chirality and shape; considering different salt bridges, and studying solvent environment under which specific results are achieved.
Metallacrowns are gaining more attention in inorganic circles. Since cavity metals with different properties can be selectively encapsulated by those rings, many applications have been proposed for metallacrowns. Trivalent lanthanide complexes, for example, have been shown to effectively hydrolyze phosphate diesters and RNA. Because the 15-MC-5 structural type encapsulates lanthanides, current work in our lab is exploring this new function.
Trivalent gadolinium is one of the lanthanides encapsulated by the metallacrowns. This cation is the most paramagentic ion known and is, therefore the best known metal used in MRI(Magentic Resonance Imaging) contrasting agents. Therefore, a new project was launched where this property of the gadolinium metallacrown is studied. It has been shown that metallacrowns have similar relaxivities to those exhibited by the MRI compounds presently in use. As a step forward in the direction of those biological and medical applications of metallacrowns, current work focusses on the stability of the 15-metallacrowns-5 in aqueous and buffered solutions at different pH's. Competition experiments with other popular complexing agents, such as EDTA, and physiologically relevant molecules, such as plasma proteins that transport transition metals in the biological systems are being investigated. The design of rings that are most stable under physiological conditions includes the synthesis of metallacrowns with inert ring metals and the study of the solution integrity exhibited by these metallacrowns.
Other applications have been proposed for the new compounds. For example, planar 12-MC-4's are similar in size and shape to porphyrins and phthalocyanines, which have been explored as liquid crystalline materials with diverse applications including data storage and directional conductivity. Metallacrowns, therefore, as liquid crystals is another possible destiny for the new class of compounds as studied by the graduate students in the lab. Also the encapsulation of specific metals, such as cerium may amplify their catalyzing properties.
Chiral metallacrowns have been fused into two-dimensional layers using neutral copper benzoate paddlewheel dimers and nitrate anions to link each 12-MC--4 molecule. (Click here for descriptive models). The solid contains approximately 9A channels that are filled with solvent and a benzoic acid guest. A related chain structure that is generated by connecting metallacrowns with the copper dimer, but which does not have nitrate bridges in the second dimension, also has channels of similar dimension. These observations suggest a generizable strategy for the preparation of mesoporous chiral solids in bulk quantities.
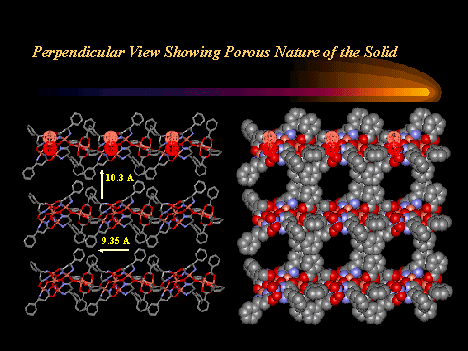
Would you like to view a movie of a layered chiral solid prepared from metallacrowns ? Click here
Metallacryptate
The metallacryptate [Mn4IIMn22III(pdol)12 (µ3-OCH3)12(µ3-O)10 (µ4-O) 6(N3)6] (pdol2-=dipyridylketonediolate ion) was synthesized by our collaborator Dr. Dimitris Kessissoglou and his students in Greece. We determined the crystal structure and its magnetic properties here in the Pecoraro group (Angew. Chem. Int. Ed. 2003, 42, 3763). The metallacryptate is basically a three-dimensional metallacrown that has an encapsulated manganese oxide core. A strand of ten Mn centers and 12 pdol2- ligands compose the outer strands and the core composition is {Mn16(O2-)12(MeO-)16}.
See the pictures below.
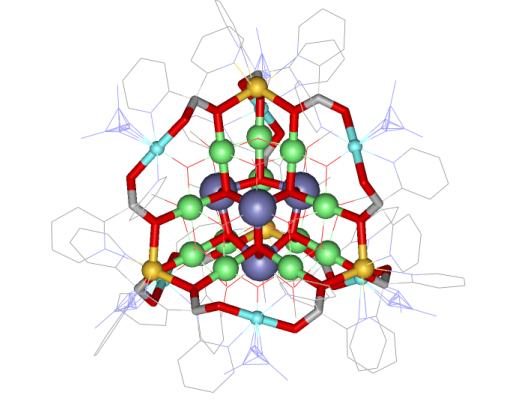
Figure 4. Complete structure of metallacryptate. The gold spheres are MnII centers, and the blue, purple, and green spheres are different crystallographic sets of MnIII centers.
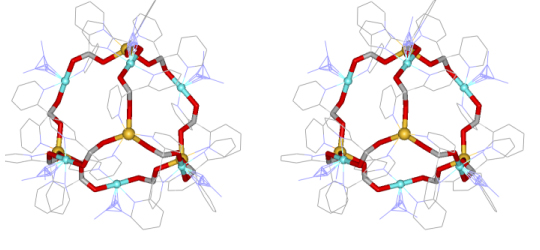
Figure 5. Stereoview of the stands of metallacryptate with the core removed. The color scheme is the same as above.
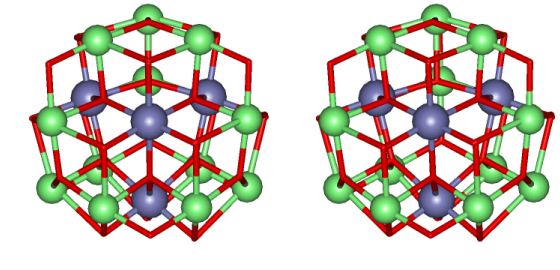
Figure 6. Stereoview of the core of the metallacryptate. Same color scheme as above.
The AC magnetic susceptibility data indicate that this molecule behaves as a single-molecule magnet (SMM). As the in-phase susceptibility decreases near 2.5 K, the out-of-phase susceptibility increases (a characteristic signal of SMM's). Unfortunately, a blocking temperature could not be determined just yet since the limit of the AC SQUID used in the study is 2.0 K. Future work at lower temperatures is in progress. The DC magnetic susceptibility data indicate that an overall antiferromagnetic interaction dominates in the cluster. However a ground state could not be determined since the magnetization does not saturate at 2K from 100 G to 55, 000 G. Work at lower temperatures and higher magnetic fields is in progress. Single-molecule magnets are a relatively new class of molecules (first identified in 1993 by Christou and coworkers) that show both classical and quantum effects and may have applications in memory storage.
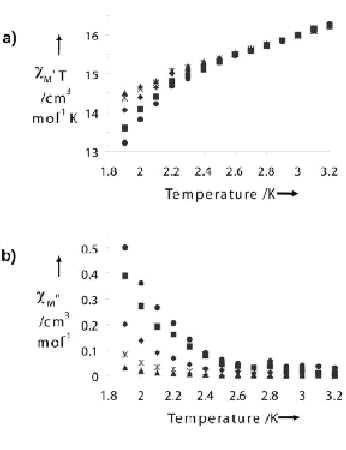
The Ac susceptibility data indicate SMM behavior.
Metallahelicate
An investigation into the use of a typical 15-MC-5 ligand to make a 12-MC-4 lead to the formation of a metallahelicate.
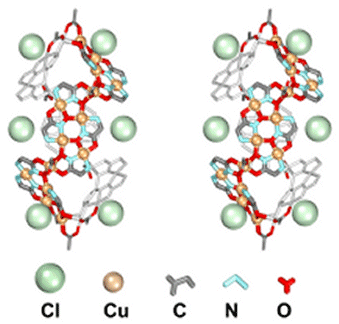
The reaction of l-norvaline hydroxamic acid, Cu(Oac)2 and NH4Cl results in a 28 copper "supermolecule" that includes 20 ligands, 10 acetate anions and 6 chloride anions, in two strands that are linked by a non-crystallographic twofold axis. This molecule is over three nanometers long, and each strand contains three structural domains; a central "body" which includes four Cu(II) and four ligands, and two identical "wings" (4Cu, 3 L) that are linked by a bridging [Cu(OAc)]+ "hinge".
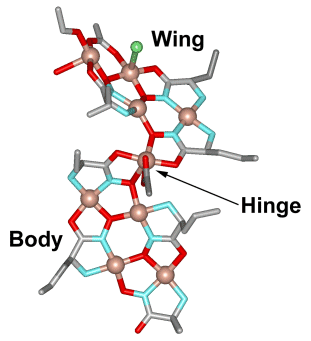
While this molecule is made from a 15-MC-5 ligand, the "body" may actually be considered to be a collapsed 12-MC-4 molecule without a central encapsulated metal. Each body unit interacts co-facially with the other, with Cu-O interactions that can be seen in the following figure.
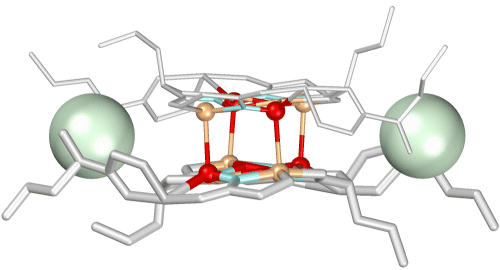
In addition, at the top and bottom of the molecule the wings of the two strands are linked by two acetate anions that bridge through coordinating to two "capping" copper sites.
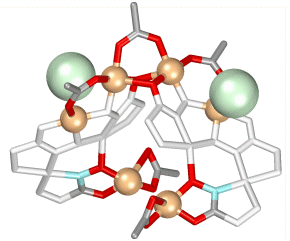
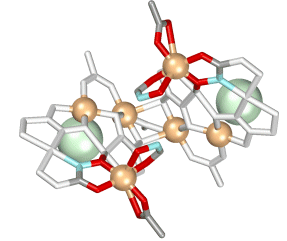
The coordination geometry around the hinge coppers is interesting, as these are 6-coordinate, and are chiral. Depending on the enantiomer of the ligand that is used to prepare the helicate, the four copper atoms have either an all ? or all ? configuration.
Advantages Offered by Metallacrowns over other organic and Inorganic Ligands
- The inclusion of transition metals in the ring gives metallacrowns spectral properties not possesed by the typical organic agents. UV-Vis, NMR and EPR techniques are now employed in the studies as well as magnetic, paramagnetic and redox properties.
- The use of metals in the rings of metallacrowns eliminates the typical structural restrictions to tetrahedral or trigonal geometries around the carbon. New geometries can be realized with the new ring incorporation.
- Metallacrowns, unlike many similar cyclic sequestering agents, such as porphyrins, texaphyrins and crown ethers, can be synthesized in one or two steps, with high yields of 40%-95%. Syntheses are carried out in common lab solvents (methanol, water, DMF(dimethylformamide)), at room temperature and are not air sensitive. That is, no extreme conditions are needed for the syntheses. Other organic and inorganic molecular recognition agents are made through multistep synthesis after which many side products contaminate the solution. Vigorous separation are needed for the isolation of these agents leading to decreased yields.
The synthesis reaction of a 15-MC-5 precursor ligand and a 15-MC-5 are shown below.
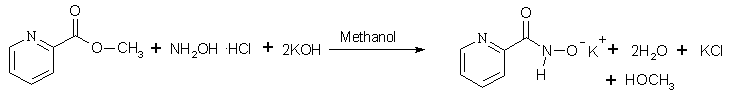
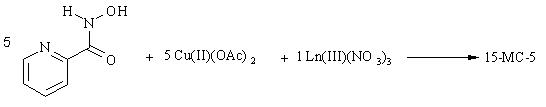
Scheme 1 The synthesis of a 15-MC-5
- The three-dimensional shape of some metallacrowns and ionic salt bridges connecting the cavity metal with the ring metal add to the stability of the ring in solution.
- Chiral 15-MC-5 can be synthesized using chiral ligands. Because of the directional nature of the metallacrowns ring, all the R-groups chiral of the chiral amino ligand used in the synthesis are forced to be on only one side of the ring , rendering the metallacrown optically active, and giving it spectral properties. As a result, CD(Cyclic Dichroism) spectra can be used to study these molecules. Appending such metallacrowns to surfaces, as an advantage can be possible. The Fe-9-MC-3 also exhibits structural chiralities at the metal centers.
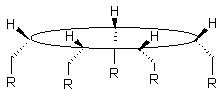
Figure 3. A chiral 15-MC-5 with a differentiated face due to the chirality of the ligand used in the synthesis.
Since metallacrowns can vary in at least four basic aspects: ring size, cavity metals, ring metals and functionalized precursor ligands, a huge amount of metallacrowns can be synthesized with different properties. A tremendous amount of applications in a wide range of fields ranging from inorganic, physical and materialistic to biological and medical functions is proposed.
Future of the Field
Our research on metallacrowns has produced a great amount of information about this new class of molecular recognition agents. As mentioned above, current projects investigate the many applications proposed for the inorganic crowns. Stability in a variety of solvents, including water, and in buffered systems is being examined. Their solution dynamics in the presence of other chelating agents and biological metal sequestering proteins is being studied. Metallacrowns are becoming part of the inorganic and bioinorganic communities as new metallacrown projects are started every year at different institutions in the country as well as around the world (in Greece, Germany, Finland and Korea). The new field has a very promising future and has many inorganic and material as well as biochemical and biological applications. Metallacrowns are indeed the wave of the future!