 = G
= G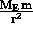 where
where
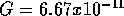
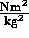 .
.
© Timothy E. Chupp, 1995
We now introduce the concept of binding energy (BE). In words, BE is the negative of the minimum work needed to separate a bound system into two pieces. Consider the ``bound system'' of the earth and a space ship at rest on its surface. The force which holds the two together, i.e. which binds the system, is of course gravity which has the magnitude
 = G
= G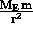 where
where
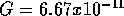
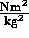 .
.
Near the earth's surface (r = r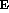 ) this is
) this is 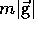 , but
as r approaches infinity, the force becomes vanishingly small.
, but
as r approaches infinity, the force becomes vanishingly small.
Now consider the space ship very far away, initially at rest. There will be a small force toward the earth and therefore it will accelerate, move closer to the earth where the force is stronger, accelerate more etc. At each point, its KE will be equal to the work done by gravity up to that point. Since the force is not constant, calculation of the work done requires the operation of summing the work for each short distance interval over which the force is approximately constant. This is called integrating the force:
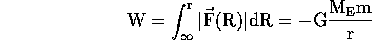
The work need to remove the space ship from r to very far away is the negative of this, and thus the
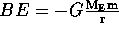
The total energy of the system is  = KE + BE. Therefore
if it is initially very far away and at rest,
= KE + BE. Therefore
if it is initially very far away and at rest,  = 0 + 0
which remains constant as the spaceship falls toward the earth.
= 0 + 0
which remains constant as the spaceship falls toward the earth.
4. If the spaceship starts at the earth's surface, what is
the binding energy of the spaceship-earth system. Express your
answer in terms of g and 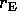 . If the total
energy of the system is 0, that is at
. If the total
energy of the system is 0, that is at 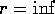 , KE=0 and BE=0, what
is the KE of the spaceship as it leaves the earths surface.
The corresponding velocity is called the escape velocity:
, KE=0 and BE=0, what
is the KE of the spaceship as it leaves the earths surface.
The corresponding velocity is called the escape velocity:
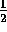 mv
mv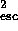 = mgr
= mgr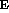
5. Find the escape velocity for a He atom at the earth's surface and compare this to the rms velocity at 300 K.
We can draw a graph of BE as a function of separation and indicate the constant
 . The KE is given by
KE =
. The KE is given by
KE =  - BE as shown.
Near the earth's surface, the change
of binding energy over small distances
is the well known
- BE as shown.
Near the earth's surface, the change
of binding energy over small distances
is the well known
 BE = mgh. This can be derived with a few lines of calculus:
BE = mgh. This can be derived with a few lines of calculus:
 BE
BE 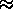
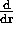 (BE)
(BE)  r
r
where
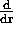 (BE) = G
(BE) = G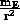 m = -mg
m = -mg
thus  BE = mg
BE = mg r = mgh.
r = mgh.
Now consider the BE of two protons. In this case, the force has magnitude given by
 = k
= k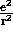 where
where
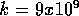
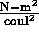 and
and 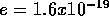 coul.
coul.
In contrast to the attractive, gravitational force, the electrical force is repulsive. Our definition of BE therefore gives
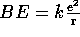
Two protons can come together only if the total energy is positive. Therefore
consider two protons greatly separated with a large 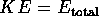 .
As they move together, BE increases and KE decreases as shown.
.
As they move together, BE increases and KE decreases as shown.
BUT two protons can come together and form a bound system through the nuclear reaction
p + p  d (pn) + e
d (pn) + e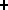 +
+ 
where d is the nucleus of deuterium (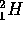 ),
the e is an electron and the
),
the e is an electron and the  is
the new particle called the neutrino which was mentioned earlier and
will soon be discussed in more detail.
In any reaction, including a nuclear reaction, energy (including mc
is
the new particle called the neutrino which was mentioned earlier and
will soon be discussed in more detail.
In any reaction, including a nuclear reaction, energy (including mc )
and electric charge must be conserved. You can easily see that this is
true for this reaction. Furthermore note that one proton has disappeared
and a neutron has appeared. Whenever this happens or the reverse, i.e.
a proton is transmuted into a neutron or vice versa, a neutrino must appear
along with the electron or positron that insures conservation of electric
charge.
)
and electric charge must be conserved. You can easily see that this is
true for this reaction. Furthermore note that one proton has disappeared
and a neutron has appeared. Whenever this happens or the reverse, i.e.
a proton is transmuted into a neutron or vice versa, a neutrino must appear
along with the electron or positron that insures conservation of electric
charge.
The nuclear fusion reaction which is better written
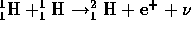 .
.
can happen because protons within about 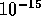 m of
each other exert nuclear forces. Nuclear forces are in fact stronger than the
repulsive electric force, but cannot be expressed in the form used for
gravitational and electric forces. In terms of BE, the nuclear binding
can be shown
by including a negative portion or well for
m of
each other exert nuclear forces. Nuclear forces are in fact stronger than the
repulsive electric force, but cannot be expressed in the form used for
gravitational and electric forces. In terms of BE, the nuclear binding
can be shown
by including a negative portion or well for 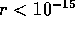 m as
shown above. In the case of deuterium, the well is 2.2 MeV deep.
(1 MeV is a unit of energy based on the electron-volt or eV. 1 eV =
m as
shown above. In the case of deuterium, the well is 2.2 MeV deep.
(1 MeV is a unit of energy based on the electron-volt or eV. 1 eV =
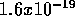 Joule, a very small amount. 1 MeV = 1 Million or
Joule, a very small amount. 1 MeV = 1 Million or 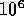 eV.
eV.
6. What is the velocity of a proton with KE=2.2 MeV?
The maximum positive BE is sometimes called the coulomb barrier which must be surmounted in order for the nuclear reaction to occur.
The curves of BE can be viewed as hills which the spaceships or protons
roll up or down. That is, a space ship above the earth at rest will
roll down the curve of BE toward the earth and a proton near another proton
will roll away down the curve of BE.
For the case of two protons, once they are within 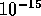 m of each other,
they will fall into the well of binding energy releasing KE which
is greater than that needed to surmount the coulomb barrier.
m of each other,
they will fall into the well of binding energy releasing KE which
is greater than that needed to surmount the coulomb barrier.
We now consider the sources of energy utilized by society. The major sources of energy now in use are gravitational energy (hydropower), chemical energy (fossil fuels), nuclear fission energy, nuclear radioactivity and solar power. Nuclear fusion energy the hypothetical source of the sun's heat, and matter-antimatter annihilation are sources which may become practical in the years to come.
In each case, we will use a figure of comparison, the useful energy avaliable
per kg of ``fuel''.
For example, hydropower is effected by building a dam of typically 100 m
height which lets 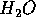 fall onto the turbines of a generator. For each
kg of
fall onto the turbines of a generator. For each
kg of 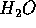 , mgh = 1000 J = 1 kJ. In this case of figure of comparison
is 1 kJ/kg. We will soon provide a basis of comparison for the
several sources of energy based on this figure of comparison
and the nature of the
waste products.
, mgh = 1000 J = 1 kJ. In this case of figure of comparison
is 1 kJ/kg. We will soon provide a basis of comparison for the
several sources of energy based on this figure of comparison
and the nature of the
waste products.
The 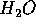 used in hydropower had to be lifted to the 100m height in order
to be useful. The agent responsible for this work is the sun which
shines on each
used in hydropower had to be lifted to the 100m height in order
to be useful. The agent responsible for this work is the sun which
shines on each 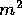 of the oceans with power of about 1 kW (1 kJ/s).
For each kg of
of the oceans with power of about 1 kW (1 kJ/s).
For each kg of 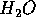 evaporated, 2,200 kJ of the sun's energy
are needed. In order to lift each kg to a height of 10 km, typical of
many clouds, only 100 kJ are needed for a total of 2,300 kJ/kg supplied
by the sun for each 1 kJ/kg exploited by hydropower. This certainly
does not seem to be an efficient use of the sun's power.
evaporated, 2,200 kJ of the sun's energy
are needed. In order to lift each kg to a height of 10 km, typical of
many clouds, only 100 kJ are needed for a total of 2,300 kJ/kg supplied
by the sun for each 1 kJ/kg exploited by hydropower. This certainly
does not seem to be an efficient use of the sun's power.
Chemical compounds are bound by electrical forces of varying strength. In breaking these bonds and forming new ones, KE is released. The burning of hydro-carbon compounds is most common. For example:
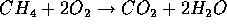
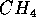 is methane abundantly produced by the metabolism of living things.
Propane,
is methane abundantly produced by the metabolism of living things.
Propane, 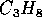 is commonly used as cooking and lab gas.
For each mole of C atoms burned, 250 kcal
is commonly used as cooking and lab gas.
For each mole of C atoms burned, 250 kcal 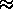 1000 kJ is released. Thus
chemical energy typically has 1000 kJ per
1000 kJ is released. Thus
chemical energy typically has 1000 kJ per 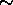 15 gm or 6x10
15 gm or 6x10 kJ/kg
to offer
as useful energy. For these simple hydro-carbons, only
kJ/kg
to offer
as useful energy. For these simple hydro-carbons, only 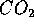 and
and 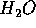 are produced as waste products. Unfortunately, most fossil fuel
is in the form of oil of varying grades of refinement and coal.
In combustion of gasoline in automobiles for example, CO (carbon monoxide)
and
are produced as waste products. Unfortunately, most fossil fuel
is in the form of oil of varying grades of refinement and coal.
In combustion of gasoline in automobiles for example, CO (carbon monoxide)
and 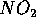 which is carcinogenic are produced due to incomplete
burning and reaction of air
which is carcinogenic are produced due to incomplete
burning and reaction of air 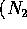 and
and 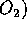 at high temperatures. Coal
is mostly carbon and sulfur. The following reactions take place
at high temperatures. Coal
is mostly carbon and sulfur. The following reactions take place
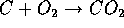 and
and
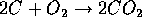
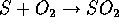 and
and
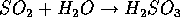 (sulfurous acid)
(sulfurous acid)
Sulfurous acid is the major component of Acid Rain. In addition, great amounts of carbon dust are produced and exhausted. Of course the emission of particulate and acid rain by products can be reduced but only at allegedly great expense to the industries which would be forced to retrofit their facilities. In the modern world, economy seems to be defined as the lowest possible cost of doing business and it seems very hard to establish the cost to the coal industry of polluting a lake in the Canadian wilderness.
Nuclear energy resides in the bonds established by the nuclear forces. Since
such forces are stronger than the electrostatic repulsion of the
positively charged protons separated by less than 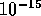 m, we can
expect at least
m, we can
expect at least 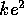 /r = 1.44 MeV per nuclear bond. In fact nuclear bonds
are as large as 8 MeV per nucleon (a nucleon is either a neutron or proton)
for the most tightly bound nuclei like
/r = 1.44 MeV per nuclear bond. In fact nuclear bonds
are as large as 8 MeV per nucleon (a nucleon is either a neutron or proton)
for the most tightly bound nuclei like 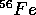 . The mass of a proton
is
. The mass of a proton
is 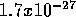 kg and nuclear fuel can therefore provide about
kg and nuclear fuel can therefore provide about
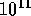 kJ/kg!
kJ/kg!
It is essential to bring a new kind of energy into the equation for
nuclear energy: this is the equivalent energy of the mass: 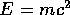 , the
famous result of Einstein's special theory of relativity.
Consider the set of nuclear reactions suggested by Hans Bethe
as the sun's primary source of energy (hydrogen fusion):
, the
famous result of Einstein's special theory of relativity.
Consider the set of nuclear reactions suggested by Hans Bethe
as the sun's primary source of energy (hydrogen fusion):
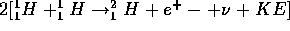
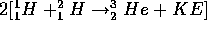
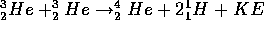
the net result of this series of reactions can be written
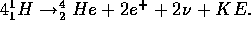
The 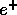 is the positively charged antiparticle of the electron called
the positron. It has the same mass but opposite electric charge as the electron.
is the positively charged antiparticle of the electron called
the positron. It has the same mass but opposite electric charge as the electron.
Even though the 4 protons must have sufficient KE to overcome the coulomb
barrier, once they are close enough, the series of reactions will occur
and provide more energy than was initially needed. This is because
the
sum of the masses of the 4 protons is greater than that of the
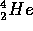 , the positrons and neutrinos. The energy conservation principle
applied to this reaction is
, the positrons and neutrinos. The energy conservation principle
applied to this reaction is
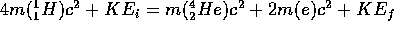 (note:
(note: 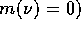
The masses of the 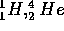 and positron are given below where 1 A.M.U.
is the atomic mass unit based on the scale for which the
and positron are given below where 1 A.M.U.
is the atomic mass unit based on the scale for which the 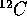 atom
has mass 12 A.M.U. (1 A.M.U. =
atom
has mass 12 A.M.U. (1 A.M.U. = 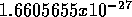 kg).
kg).
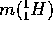 = 1.00782 A.M.U.
= 1.00782 A.M.U.
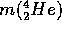 = 4.002596 A.M.U.
= 4.002596 A.M.U.
m(e) = 0.0005485
Applying this to the energy conservation equation, we find
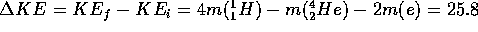 MeV
(0.0276 A.M.U.)
MeV
(0.0276 A.M.U.)
This set of fusion reactions is suggested as the sun's primary source of energy. This suggestion is a hard one to test, but physicists have been working for two decades to detect the neutrinos that are produced by the nuclear fusion reactions. These are the only particles that reach the earthbound observatory unaffected, i.e. they pass through the sun with very little probability of interacting. (This statement, believed valid in 1985, may reversed by 1987.) For the same reason, neutrinos are very hard to detect, in fact a volume of material several light years thick would be required to insure that an incident neutrino interacts. I have not written the complete set of nuclear fusion reactions which have the final result that four protons become one helium, positrons and neutrinos. It turns out that the currently detectable neutrinos do not come from the set of reactions written above but from a set of reactions that take place less than 1% of the time.
The 26.6 MeV avaliable for each 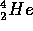 atom formed is
atom formed is 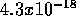 J.
Therefore for each Joule of radiant energy (sunshine)
J.
Therefore for each Joule of radiant energy (sunshine) 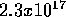
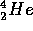 atoms are formed. We can use this to predict two numbers: how long the
sun can shine and how many neutrinos are currently incident on your head.
For the first, you need to know the sun's mass,
atoms are formed. We can use this to predict two numbers: how long the
sun can shine and how many neutrinos are currently incident on your head.
For the first, you need to know the sun's mass, 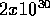 kg and that
75% of this mass is avaliable as
kg and that
75% of this mass is avaliable as 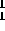 H fuel. For the second, use the fact
that on a sunny day, about 1 kW shines on each
H fuel. For the second, use the fact
that on a sunny day, about 1 kW shines on each 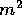 of the earth.
of the earth.
Hydrogen fusion may provide useful power for society. The challenge is to maintain a controlled fusion reaction, that is to contain a sufficient amount of the energy produced as heat, the KE of the protons which is necessary to surmount the coulomb barrier. This work is actively pursued especially at Princeton, MIT and the US National Laboratories at Livermore and Los Alamos. Though great progress has been made, the utilization of controlled hydrogen fusion is probably decades in the future.
Hydrogen fusion is exploited in a non-controllable way in the Teller-Ulam hydrogen bomb which has formed the centerpiece of US and Soviet nuclear arsenals since the mid 1950's.
The hydrogen bomb consists of a fission bomb (we will discuss the fission reactions soon), triggers, deuterium and hydrogen as fuels and a jacket of uranium which contains the energy of the fission bomb's explosion long enough to ignite the fusion fuel. It is a complex but compact device whose energy content is measured in kilotons or megatons of TNT, i.e. the energy avaliable from the equivalent chemical explosion:
1 ton = 1000 kg which provides 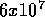 kJ of chemical energy
kJ of chemical energy
Typical U.S. bombs are 100 kilotons and typical Soviet bombs are 1 Megaton. All together, the combined arsenals of the world contain at least 10,000 such devices.
Nuclear Fission
Nuclear fission is the process of a heavy nucleus (Z>90) breaking up into two
roughly equal parts and possibly individual neutrons. In particular,
the nuclei 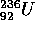 and
and 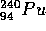 (U is Uranium and
Pu is Plutonium) break up as follows:
(U is Uranium and
Pu is Plutonium) break up as follows:
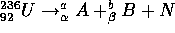 neutrons,
neutrons,
and similarly for 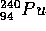 . The products of fission for a single
nucleus are not definitely predictable. N, the number of neutrons
produced has an average value of 2.3.
A and B are elements in the region of
. The products of fission for a single
nucleus are not definitely predictable. N, the number of neutrons
produced has an average value of 2.3.
A and B are elements in the region of  and
and 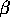 = 45-56
i.e. Rh, Pd, Ag, Cd, In, Sn, Sb, Te, I, Xe, Cs and Ba with
mass a and b between 80 and 160. Conservation of mass (energy)
and charge (Z) require for
= 45-56
i.e. Rh, Pd, Ag, Cd, In, Sn, Sb, Te, I, Xe, Cs and Ba with
mass a and b between 80 and 160. Conservation of mass (energy)
and charge (Z) require for 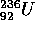 that
that 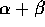 = 92
and a + b + N = 236.
= 92
and a + b + N = 236.
The reason that these nuclei fission can be understood by considering
the curve of binding energy which is shown for 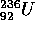 .
.
The nucleus in its bound state (the well to the right of the hump or barrier)
is relatively stable, the half life is 24 million years. However if
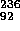 U is formed by the reaction
U is formed by the reaction
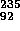 U + n (neutron)
U + n (neutron) 
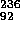 U
U
the product nucleus 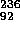 U will have sufficient excitation
to surmount the barrier and break into the fission products. This
excitation is due to the KE of the individual neutrons and protons
in the nucleus and is analogous to the internal energy associated with the heat
of a macroscopic object. When the nucleus fissions, the entire barrier energy,
210 MeV, becomes avaliable as the KE of the fission products. This is
about 1 MeV per neutron or proton i.e. 10
U will have sufficient excitation
to surmount the barrier and break into the fission products. This
excitation is due to the KE of the individual neutrons and protons
in the nucleus and is analogous to the internal energy associated with the heat
of a macroscopic object. When the nucleus fissions, the entire barrier energy,
210 MeV, becomes avaliable as the KE of the fission products. This is
about 1 MeV per neutron or proton i.e. 10 kJ/kg!
kJ/kg!
The reaction written above is the basis for the uranium fission bomb. The
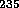 U is found in nature today as 0.7%
of the uranium,
and the other 99.3% of natural uranium is
U is found in nature today as 0.7%
of the uranium,
and the other 99.3% of natural uranium is 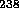 U.
Uranium was present when the earth was formed.
U.
Uranium was present when the earth was formed. 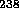 U can also
capture a neutron to form
U can also
capture a neutron to form 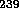 U which may fission, but this system is
more tightly bound i.e. the well is deeper. Therefore higher energy
neutrons are necessary (such as those produced in a thermonuclear bomb which
cause fission of the
U which may fission, but this system is
more tightly bound i.e. the well is deeper. Therefore higher energy
neutrons are necessary (such as those produced in a thermonuclear bomb which
cause fission of the 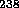 U jacket shown on page 41).
The probability that a neutron will be captured and therefore that the fission
will occur is proportional to 1/v, the inverse of the neutron's velocity.
U jacket shown on page 41).
The probability that a neutron will be captured and therefore that the fission
will occur is proportional to 1/v, the inverse of the neutron's velocity.
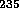 U and
U and 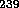 Pu
which can fission with lower energy or slow neutrons is much more effective
for a fission bomb.
Pu
which can fission with lower energy or slow neutrons is much more effective
for a fission bomb.
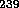 Pu does not occur naturally. It is formed in reactors which
have some
Pu does not occur naturally. It is formed in reactors which
have some  U through the series of reactions
U through the series of reactions
 U + n
U + n 
 U (23.5 min)
U (23.5 min)
 U
U 
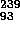 Np (2.35 day) + e
Np (2.35 day) + e +
+ 
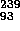 Np
Np 
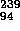 Pu (24,000 yr) + e
Pu (24,000 yr) + e +
+ 
The times in parenthesis are the half-lives of each product.
The half-life of 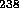 U is 4.5 billion years, about the age of the earth.
The half-life of
U is 4.5 billion years, about the age of the earth.
The half-life of 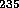 U is 800 million years.
U is 800 million years.
A Short History
Fission was discovered in the laboratory of Otto Hahn and Fritz Strassmann in Germany in 1938. This was followed by the explanation of Lise Meitner which served to clear up some of the mysteries surrounding results of bombarding heavy, radioactive materials such as radium and uranium with neutrons. The realization that much lighter elements were produced (Hahn and Strassmann identified Ba) along with more neutrons and energies typical of nuclear reactions was rapidly synthesized into the realization that a weapon of unprecedented explosive energy was possible. This explosion would be due to a chain reaction leading to exponential growth of the fission rate and the energy produced until the fuel is spent.
The discovery of fission may have been the final ``peace-time'' discovery of the young field of nuclear physics which was born in 1896 with the discovery by Bequerell that certain uranium salts caused a blackening of photographic film. The following list highlights some of the events before 1938:
1896 - Becquerel discovers radioactivity.
1897-99 - J.J. Thompson's investigations show the existence of the electron.
1898 - E. Rutherford discovers  rays in Rn gas and deflects them
magnetically to contrast with
rays in Rn gas and deflects them
magnetically to contrast with 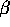 rays.
rays.
1900 - 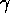 radiation was discovered by Villard - it is more difficult to
shield than
radiation was discovered by Villard - it is more difficult to
shield than 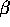 radiation.
radiation.
1903 - 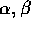 and
and 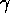 radiation are all shown to cause
transmutation, that is changing the chemical form of an atom.
radiation are all shown to cause
transmutation, that is changing the chemical form of an atom.
1903 - Rutherford shows  rays are
rays are  He nuclei.
He nuclei.
1911 - E. Rutherford scatters  particles from gold atoms
(1904 - 1908) and
discovers that some particles are scattered back toward the source as
if a strongly repulsive object was a the center of the atom. This was
of course the positively charged nucleus and gave way to Rutherford's
model of the atom as a cloud of electrons surrounding a nucleus of positive
charge.
particles from gold atoms
(1904 - 1908) and
discovers that some particles are scattered back toward the source as
if a strongly repulsive object was a the center of the atom. This was
of course the positively charged nucleus and gave way to Rutherford's
model of the atom as a cloud of electrons surrounding a nucleus of positive
charge.
1919 - Rutherford observes positively charged, longer ranged particles that
 s - protons. This was achieved by exposing nitrogen to
s - protons. This was achieved by exposing nitrogen to  particles from radium and was the first artificially induced radioactivity.
particles from radium and was the first artificially induced radioactivity.
1932 - The neutron was discovered by Chadwick
bombarding 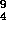 Be with alpha particles and observing
Be with alpha particles and observing 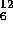 C as
the product. Charge conservation along with energy and momentum
conservation require the existence of a neutral particle with a mass
similar to the protons: the neutron.
C as
the product. Charge conservation along with energy and momentum
conservation require the existence of a neutral particle with a mass
similar to the protons: the neutron.
1932 - Cockroft and Walton built the first high voltage accelerator which
could give protons and other positive projectiles sufficient energy to
surmount the coulomb barrier and undergo nuclear reactions. The observed
reaction was 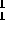 H +
H + 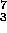 Li
Li  2
2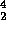 He.
Later that year
Ernest Lawrence at Berkeley developed the cyclotron and observed the same
reaction.
He.
Later that year
Ernest Lawrence at Berkeley developed the cyclotron and observed the same
reaction.
1932-38 Many elements were bombarded with  particles and neutrons
in the search for new elements. Those heavier than uranium were
named transuranic elements. This new kind of alchemy produced new mysteries
including that associated with bombarding
particles and neutrons
in the search for new elements. Those heavier than uranium were
named transuranic elements. This new kind of alchemy produced new mysteries
including that associated with bombarding 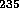 U. Meitner's suggestion
that fission occurred set into
motion the development of nuclear weapons, the first chapter of which
ended in the summer of 1945.
U. Meitner's suggestion
that fission occurred set into
motion the development of nuclear weapons, the first chapter of which
ended in the summer of 1945.
Developing Atomic Energy During the War
During the 1930's a great many European scientists, mostly Jewish, came to the U.S. fleeing from the political and social situations in their homelands. The list of names is long and well documented in the narrative by Laura Fermi Illustrious Immigrants published in 1968. Some key figures were H. Bethe, A. Einstein, E. Fermi, G. Gamow, G. Kistiakowsky, L.Szilard, E. Teller, S. Ulam, E. Wigner, V. Weisskopf and many others. The highlights of the birth of nuclear physics show that before 1940, physics research was concentrated in Europe. The birth and nurturing of Quantum Mechanics, the basis of modern physical thought, paralleled that of nuclear physics. The center of its development was the institute of Niels Bohr in Copenhagen.
With the scientists' emigration came the new ideas of physics as well as new channels of communication to European research. In particular, news of the suggestion of Meitner and its experimental confirmation quickly found its way to the recently landed Leo Szilard. Together with E. Wigner, Szilard mounted a campaign to bring the weapons potential of nuclear fission to the attention of F.D. Roosevelt, the U.S. president. They enlisted Einstein as a co-signer of a letter to Roosevelt in the hope that the name of the famous physicist would bring more rapid attention. This was in the summer of 1939, fully 18 months before the US declared war on Germany and Japan. A great deal of research took place during those 18 months but the US decision to make an all out effort to exploit nuclear fusion came in early December, 1941, just before the bombing of Pearl Harbor on December 7.
The work of the Manhattan Project took place on many fronts for there were
many problems to solve. First came
the question of how much material was needed
for a bomb: the fission chain reaction would take place only if the
neutrons produced in a single fission reaction had sufficient probability
to be absorbed and beget another fission reaction. In fact, on the average,
each neutron absorbed must lead to the fission of more than one more atom in
order for
the chain reaction to grow. The probability of neutron absorption depends
on the density of the absorbers, the 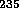 U or
U or 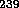 Pu. A sufficiently
high density for an explosion is called the critical density
which is often misnamed the critical mass.
Only a few kg of fissionable material is needed for a weapon.
The study of neutron absorption
probability on
Pu. A sufficiently
high density for an explosion is called the critical density
which is often misnamed the critical mass.
Only a few kg of fissionable material is needed for a weapon.
The study of neutron absorption
probability on 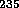 U and
U and 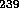 Pu took place at laboratories in
Chicago and Berkeley among others.
Another problem was producing the fuel,
i.e. extracting the 0.7%
Pu took place at laboratories in
Chicago and Berkeley among others.
Another problem was producing the fuel,
i.e. extracting the 0.7% 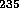 U in natural uranium and producing
U in natural uranium and producing
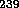 Pu. Oak Ridge Laboratory in Tennessee and Hanford in Washington State
became central in this effort. The critical density would be achieved
by a compressive explosion which was developed in part at Los Alamos, New
Mexico which, under the direction of J.R. Oppenheimer,
became the center of the wartime research and the site
of the bombs' construction.
These efforts all came together by the summer of 1945, after the surrender
of the Axis in Europe, but while the war with Japan in the Pacific was
still raging. By mid July, three bombs had been constructed, two with
Pu. Oak Ridge Laboratory in Tennessee and Hanford in Washington State
became central in this effort. The critical density would be achieved
by a compressive explosion which was developed in part at Los Alamos, New
Mexico which, under the direction of J.R. Oppenheimer,
became the center of the wartime research and the site
of the bombs' construction.
These efforts all came together by the summer of 1945, after the surrender
of the Axis in Europe, but while the war with Japan in the Pacific was
still raging. By mid July, three bombs had been constructed, two with
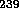 Pu and one with
Pu and one with 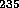 U. On July 16, 1945 at 5:30 A.M. at Alamogordo,
N.M., the first atomic bomb built by man was exploded. The intensity of the
blast took by surprise even those who had calculated its potential, for
such a physical experience cannot be equated with a number on a piece of paper.
(If I were to show you a spark 200 times larger than one you had seen
previously, you would not really know what to expect. The Alamogordo Test
was equivalent to simultaneously exploding about 20,000 tons of
TNT. In a previous test, 100 tons of TNT were exploded.)
But the carnage at Hiroshima and its after effects were a sort
of destruction that even the experience of the Alamogordo test could not
foretell.
U. On July 16, 1945 at 5:30 A.M. at Alamogordo,
N.M., the first atomic bomb built by man was exploded. The intensity of the
blast took by surprise even those who had calculated its potential, for
such a physical experience cannot be equated with a number on a piece of paper.
(If I were to show you a spark 200 times larger than one you had seen
previously, you would not really know what to expect. The Alamogordo Test
was equivalent to simultaneously exploding about 20,000 tons of
TNT. In a previous test, 100 tons of TNT were exploded.)
But the carnage at Hiroshima and its after effects were a sort
of destruction that even the experience of the Alamogordo test could not
foretell.
The decision of how to use the two remaining bombs lay with the military and ultimately with President Truman. However four of the physicists who led the efforts to produce the weapons were honored with the opportunity to advise the President. They were Arthur Compton, Oppenheimer, E.O. Lawrence and Enrico Fermi. A seriously debated option was to stage a demonstration of the weapon's destructive power for the Japanese Emperor and military leaders. This was probably the last time scientists have been involved in the formation of nuclear weapon policy, though Szilard in particular, foreseeing the proliferation of nuclear weapons campaigned strongly to prevent the consolidation to the military of atomic energy development.
On August 6, 1945, a bomb of 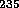 U was exploded over Hiroshima and
on August 9, 1945, one of
U was exploded over Hiroshima and
on August 9, 1945, one of 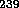 Pu was exploded over Nagasaki. All three
of the first atomic bombs built worked. There were no duds. Nor
were there any nuclear weapons in the world on August 10. Today there
are more than 10,000 most of which are controlled by the US and USSR.
Britain, France, India, China and probably Israel are also members of this
Nuclear Club, and other, developing nations are aspiring to become members.
It is interesting to note that Japan, certainly technologically capable
of producing nuclear weapons will not do so.
Pu was exploded over Nagasaki. All three
of the first atomic bombs built worked. There were no duds. Nor
were there any nuclear weapons in the world on August 10. Today there
are more than 10,000 most of which are controlled by the US and USSR.
Britain, France, India, China and probably Israel are also members of this
Nuclear Club, and other, developing nations are aspiring to become members.
It is interesting to note that Japan, certainly technologically capable
of producing nuclear weapons will not do so.
The Aftermath
The events since 1945 can be quickly summarized. The highlights are
the development of the thermonuclear or hydrogen bomb in the US in
1952 and shortly thereafter in the USSR, the deployment of nuclear
weapons on long-range bombers (B-52's), intercontinental ballistic missiles
(ICBM's), submarines (SLBM's), cruise missiles (ALCM's etc.) and
of course the development of commercial nuclear power in the US
and other countries since the 1960's. One final note which relates the
peaceful application of atomic energy in power production and weapons:
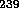 Pu is most abundantly produced in reactors as a waste product.
It is both poisonous and of course a weapons fuel.
Pu is most abundantly produced in reactors as a waste product.
It is both poisonous and of course a weapons fuel.
We have come sufficiently far in our discussion to produce the table which compares the different sources of energy. We use two bases of comparison: the energy avaliable per kg and the waste products. The two entries at the end of the table: nuclear fusion and matter-antimatter conversion are not practical though research is in progress on both fronts (the matter-antimatter work is pursued by, among others, the US Air Force).
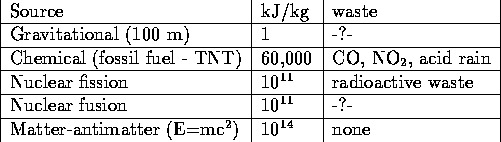
The -?- are entered because the anticipated waste products are negligible compared to those for chemical and nuclear fission power. Radioactive waste will be discussed in the next section.
Conspicuously missing from this table is direct solar power.
(Of course all of these sources of energy indirectly rely on
solar or stellar energy.)
Solar power has found practical use for home heating in some climates
and certainly reduces the dependence on the other commercial power
sources in any climate. For electrical power generation, solar energy
is not yet competitive because the energy required to construct
a device that harnesses this energy is greater than the energy the
device can produce during its practical life. Research continues on
this front with the most promise being the efficient conversion of solar to
chemical energy, e.g. the separation by electrolysis of H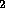 O into
H
O into
H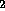 and O
and O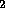 .
.
There are three types of nuclear radioactivity:  ,
, 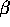 and
and 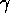 .
These are due to different kinds of nuclear decay and the
.
These are due to different kinds of nuclear decay and the  rays
etc. are particles emitted when a nucleus decays. Each of these types of
radiation
has unique characteristics, but one thing all have in common is the result of
passing through matter: they ionize the atoms in the material through which they
pass, losing kinetic energy. Ionization is the process of tearing electrons
away from the material's atoms and occurs due to the strong electrical forces.
It is ionization that leads to the harmful effects of radiation to be discussed
soon.
rays
etc. are particles emitted when a nucleus decays. Each of these types of
radiation
has unique characteristics, but one thing all have in common is the result of
passing through matter: they ionize the atoms in the material through which they
pass, losing kinetic energy. Ionization is the process of tearing electrons
away from the material's atoms and occurs due to the strong electrical forces.
It is ionization that leads to the harmful effects of radiation to be discussed
soon.
 decay
decay
Alpha decay is the emission of a 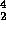 He nucleus:
He nucleus:
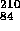 Po
Po 
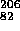 Pb +
Pb + 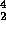 He
He
Note that this reaction conserves both Z and A, respectively the subscript and
superscript on the elemental symbol.  decay takes place
primarily with heavy elements in the uranium, thorium and actinium series.
decay takes place
primarily with heavy elements in the uranium, thorium and actinium series.
The range of  particles in matter is very short, a fraction of
a millimeter. This is the reason that a single playing card or piece
of paper is effective shielding of
particles in matter is very short, a fraction of
a millimeter. This is the reason that a single playing card or piece
of paper is effective shielding of  s. This is also the reason
that
s. This is also the reason
that  radiation is particularly biologically harmful: the
entire KE is deposited in a small volume.
radiation is particularly biologically harmful: the
entire KE is deposited in a small volume.
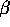 decay
decay
Beta decay occurs when a neutron within the nucleus transforms into a proton:
n  p + e
p + e +
+ 
The neutrino ( ) always accompanies such a process. Note that charge is
conserved as expected. The decay of the free neutron is a process that
occurs in nature with a half-life of about 900 s (15 min). Free protons
cannot decay due to energy conservation since mc
) always accompanies such a process. Note that charge is
conserved as expected. The decay of the free neutron is a process that
occurs in nature with a half-life of about 900 s (15 min). Free protons
cannot decay due to energy conservation since mc for the proton is less than that for the neutron.
However their are nuclei which are neutron deficient ( i.e. they would
prefer
more neutrons) for which positron decay or the process of capturing atomic
electrons can occur:
for the proton is less than that for the neutron.
However their are nuclei which are neutron deficient ( i.e. they would
prefer
more neutrons) for which positron decay or the process of capturing atomic
electrons can occur:
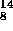 O
O 
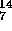 N + e
N + e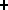 +
+  (positron decay)
(positron decay)
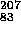 Bi + e
Bi + e

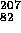 Pb +
Pb +  (electron capture)
(electron capture)
The electrons have much longer range than  particles and are often
called minimum ionizing particles.
However when they do slow down in matter, they abundantly produce X-rays
called brehmsstrahlung. This is the principle of the X-ray machines used
so commonly in dental and medical work.
particles and are often
called minimum ionizing particles.
However when they do slow down in matter, they abundantly produce X-rays
called brehmsstrahlung. This is the principle of the X-ray machines used
so commonly in dental and medical work.
 radiation
radiation
Gamma radiation arises when a nucleus is left in an excited state, usually
following 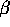 decay. The nucleus is a bound system, and like an atom
it has energy levels. Transitions among the energy levels require that
energy be removed or added to the nucleus. When an excited state decays
to the ground state or a lower energy excited state, a specific amount
of energy is released in the form of a
decay. The nucleus is a bound system, and like an atom
it has energy levels. Transitions among the energy levels require that
energy be removed or added to the nucleus. When an excited state decays
to the ground state or a lower energy excited state, a specific amount
of energy is released in the form of a 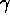 -ray. The
-ray. The 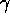 -ray
has particle-like properties, in particular it carries momentum as well
as energy, but it is a form of electromagnetic energy just as radio waves,
light and X-rays are.
The
-ray
has particle-like properties, in particular it carries momentum as well
as energy, but it is a form of electromagnetic energy just as radio waves,
light and X-rays are.
The 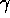 -ray carries the signature of the nucleus from
which it was emitted. The sodium-iodide detector and pulse-height
analyzer shown in lecture can be used to identify
the source of
-ray carries the signature of the nucleus from
which it was emitted. The sodium-iodide detector and pulse-height
analyzer shown in lecture can be used to identify
the source of  -rays and identify the material in a substance.
-rays and identify the material in a substance.
Gamma-rays are minimum ionizing particles. Their major interaction with matter is that of knocking electrons out of the atoms. These electrons can leave the material, ionize atoms in the material and produce brehmsstrahlung.
Nuclear radiation can be harmful to life. Of course there is a natural
background to which we are all exposed. The major components of this background
are the radioactive isotopes present in the material around us ( e.g.
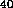 K and uranium) and cosmic-rays, particles that bombard the earth's
atmosphere from space. The atmosphere at sea level is much more effective
as shielding than that at the altitude of Denver or at 10 km, the altitude
of commercial airline traffic.
K and uranium) and cosmic-rays, particles that bombard the earth's
atmosphere from space. The atmosphere at sea level is much more effective
as shielding than that at the altitude of Denver or at 10 km, the altitude
of commercial airline traffic.
A system of units has emerged from the understanding of the harmful effects. These units became news with the recent accident in the USSR.
Curie - This is a unit of activity: 1 Cu = 3.7x10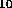 disintegrations
per second.
disintegrations
per second.
rad (radiation absorbed dose) - This is a measure of the amount of energy
deposited in a given mass of e.g. tissue or H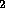 O: 1 rad = 0.01 J/kg.
O: 1 rad = 0.01 J/kg.
rem (equivalent dose) - This takes into account the different effective harmfulness of different kinds of radiation through a factor Q once called RBE for relative biological effectiveness.

For 1 rad of electrons, you have 1 rem. For 1 rad of  s you have
20 rem. The much greater RBE for
s you have
20 rem. The much greater RBE for  s etc. is due to the fact the
energy is deposited in a much smaller volume.
s etc. is due to the fact the
energy is deposited in a much smaller volume.
Exposure to radiation depends on the rate at which the ionization is produced. Federal and state regulations set limits on both the rate and integrated dose. For Massachusetts, the limits are
1250 millirem per 3 months or up to 3 rem per Quarter but less than 5 rem per year
10% of this
The harmful result of radiation exposure follows from the ionization of biological material, mostly water.
H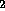 O + radiation
O + radiation  H
H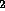 O
O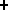 + e
+ e
These ions quickly interact forming the radicals
OH, H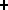 , H and OH
, H and OH , H
, H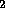 O
O (hydrogen peroxide). These newly formed radicals
interact with the atoms of the cell and chromosomes, breaking long chains
etc. The result of all of this may be:
(hydrogen peroxide). These newly formed radicals
interact with the atoms of the cell and chromosomes, breaking long chains
etc. The result of all of this may be:
- early death for the cell
- prevention or delay of cell division
- genetic modification which is passed on to subsequent cell generations
The results may be rapid onset of radiation sickness as the cells are
poisoned, mutant, cancer causing cell genesis and genetic damage that
is passed on to future generations. The most frequent cancers appear
to be leukemia, cancer of the red bone marrow and lung cancer and
breast cancer among women. Thyroid cancer and bone sarcoma occur
at a lower rate per dose. Data show that for exposure of 1 million
people to 100 millirem (10 man-rem), 10 men and 15 women will
contract cancers.
man-rem), 10 men and 15 women will
contract cancers.
In fact knowledge of the relationship of exposure and its harmful effects rests on sparse data collected mostly in the aftermath of Hiroshima and Nagasaki and accidental exposures. It is on these data, however, that regulations are set.
Figure 1: Two dimensional elastic collisions of two equal mass objects.
Physics 125
This document was generated using the LaTeX2HTML translator Version 95.1 (Fri Jan 20 1995) Copyright © 1993, 1994, Nikos Drakos, Computer Based Learning Unit, University of Leeds.
The command line arguments were:
latex2html -split 0 -link 0 -no_navigation sixt.tex.
The translation was initiated by Jill Boltman on Fri Oct 27 15:41:11 EDT 1995