Physics 125
©Timothy E. Chupp, 1995
The term fluids applies to both liquids and gases.
the liquid state is less tightly bound than the solid state of the a
substance. Liquids generally take the shape of their container, but
are subject to the forces due to surface tension. Most liquids can
be treated as incompressible, that is the density depends only
slightly on applied pressure. And liquids have viscosity, a kind
of friction that produces drag in a moving fluid or on an object moving
through a liquid.
A gas is a collection of essentially unbound molecules. A gas is
compressible, that is the density can be readily changed by applying
pressure. Gases can be contained but do not have appreciable surface
tension.
The pressure in a fluid is the normal force the fluid applies on any surface
such as the container walls. Consider a flat bottomed glass. When the glass
is empty, or more accurately filled with air, the force the bottom of the
glass exerts on the air is equal in magnitude to the force that
the entire column of
air exerts on the bottom of the glass: 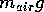 . To demonstrate this,
place a piece of newspaper on top of a meter stick or rod on a table. Try to
rapidly lift the paper by way of the rod. You will meet resistance
because, in essence, you must lift a column of air many kilometers
high. In fact, the air squishes around and the actual result of your
experience will depend on how fast you try to lift the rod, but
the meaning of pressure should be obvious.
. To demonstrate this,
place a piece of newspaper on top of a meter stick or rod on a table. Try to
rapidly lift the paper by way of the rod. You will meet resistance
because, in essence, you must lift a column of air many kilometers
high. In fact, the air squishes around and the actual result of your
experience will depend on how fast you try to lift the rod, but
the meaning of pressure should be obvious.
The mass of the column of air above the glass is not uniform in
density. In fact it is most dense at the surface of the earth.
As the altitude increases, the density of the air decreases
exponentially. At an altitdude of 10 km, about the height of
Mount Everest the pressure of air is about 28% (210 mm of Hg)
and at 20 km the air pressure is 5.5% (42 mm Hg) of that at sea level.
The air pressure at sea level is about 10 Pascals
(Pa). The unit of pressure is Pascals (Pa) where 1 Pa = 1 N/m
Pascals
(Pa). The unit of pressure is Pascals (Pa) where 1 Pa = 1 N/m .
.
Another way to be convinced of the pressure of the air is to use a manometer.
This device is a U-tube with an incompressible liquid in it, often
mercury (Hg). If both ends of the U-tube are open to the atmosphere,
the level of Hg is equal on the two sides. We can evacuate one side
of the U-tube so that there is no air pressure. The level of Hg
is higher on the evacuated side by about 0.76 m (760 mm or about
30 inches). This column of Hg is supported by the force of the
air on the side open to the atmosphere. The force is given by the
density and volume of Hg:
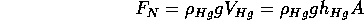
where

is the cross sectional area of the tube. The pressure of the
atmosphere is
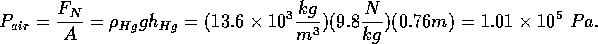
Now consider an addditional mass of water sitting on the bottom of the glass.
That is, fill the glass. The force exerted on the glass is increased
by an amount equal to

. The pressure is the normal
force per unit area:
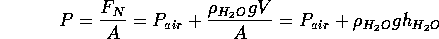
The expression for the extra pressure due to the water is meaningful
if water is an incompressible fluid, that is when

is
a constant. The air is not incompressible. The deeper the water, the
greater the pressure. This is common experience. At 3 meters, about
10 feet, below the surface of a swimming pool, the pressure exceeds
atmospheric pressure by
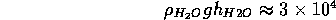
Pa. You can definitely feel this in your ears before equalizing pressure
by blowing with your nose plugged.
The term ``gauge pressure'' corresponds to the pressure above
normal atmospheric pressure, that is

. Thus
gauge pressure of the atmosphere at sea level is ZERO, and
gauge pressure at a depth of 3 meters is 30 kPa.
Try this: put a drinking straw into a cup full of your favorite drink.
Seal the top of the straw with your finger, and lift the straw out of the
cup. Note that the level of liquid in the straw remains. Why? This
is how a pipette works.
A remarkable aspect of fluids is that the pressure is uniformly distributed
throughout the fluid. Thus the force exerted on the walls of a fluid
filled container is related to the pressure by

where

is the force exerted normal or perpendicular to the surface
of area

. This, combinded with their relative incompressibility, makes
fluids amazingly useful in hydraulics. Consider a device made up of a
tube with very small cross section at one end and very large cross section
at the other end. An excess pressure

at the small end requires a
normal force

At the large end, this can produce a force
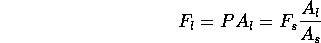
Thus the ratio of cross sections

provides a mechanical advantage
that is often utilized, for example in an automobile lift.
When an object is placed in a contianer filled with fluid, the fluid
exerts a force on the object due to the pressure. Consider a flat bottomed
boat that floats on water. The water exerts an upward force proportional
to the are of the boat's bottom and to the pressure at the bottom of the
boat, 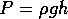 . Therefore the upward force is proportional to
the product of the area and the depth or draught of the boat, that is
the upward force is proportional to the volume of the
water displaced by the boat. The force is equal to
. Therefore the upward force is proportional to
the product of the area and the depth or draught of the boat, that is
the upward force is proportional to the volume of the
water displaced by the boat. The force is equal to 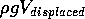 ,
that is it is equal to the ``weight'' of the fluid displaced.
,
that is it is equal to the ``weight'' of the fluid displaced.
Such buoyant forces exist for all fluids, that is for liquids and gases.
A helium filled balloon is buoyed upward because the total weight of the
filled balloon is
less that the weight of the air displaced. A boat floats because the
weight of the water displaced is equal to the weight of the boat.
Sunken objects, that is objects that weight more than the fluid displaced,
are subject to buoyant forces. Consider this: when an anchor is thrown out of
a boat, does the level of water in the lake go up or down?

Liquids are characterized by cohesive forces due to interatomic
forces. In the bulk of the liquid these forces lead to incompressibility and
viscosity. At the interface between a liquid and some other material
(solid, liquid or gas) another kind of force exists that is a manifestation
of the two dimensional nature of the surface. Surface tension is a
force per unit length. A soap bubble forms on a hoop because of the forces
between the liquid soap and the material of the hoop.
Positive surface tension indicates that the force between a surface and
substrate is attractive. In some cases, a fluid is repelled from a substrate
and the surface tension is negative. Capillary action results from positive
surface tension. The meniscus formed between water and a glass, or between
mercury and a glass is due to surface tension.
Physics 125
This document was generated using the LaTeX2HTML translator Version 0.6.4 (Tues Aug 30 1994) Copyright © 1993, 1994, Nikos Drakos, Computer Based Learning Unit, University of Leeds.
The command line arguments were:
latex2html -link 0 -split 0 -no_navigation tone.tex.
The translation was initiated by Scott D. Dexter on Tue Dec 5 16:30:45 EST 1995
Scott D. Dexter
Tue Dec 5 16:30:45 EST 1995
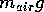 . To demonstrate this,
place a piece of newspaper on top of a meter stick or rod on a table. Try to
rapidly lift the paper by way of the rod. You will meet resistance
because, in essence, you must lift a column of air many kilometers
high. In fact, the air squishes around and the actual result of your
experience will depend on how fast you try to lift the rod, but
the meaning of pressure should be obvious.
. To demonstrate this,
place a piece of newspaper on top of a meter stick or rod on a table. Try to
rapidly lift the paper by way of the rod. You will meet resistance
because, in essence, you must lift a column of air many kilometers
high. In fact, the air squishes around and the actual result of your
experience will depend on how fast you try to lift the rod, but
the meaning of pressure should be obvious.
 Pascals
(Pa). The unit of pressure is Pascals (Pa) where 1 Pa = 1 N/m
Pascals
(Pa). The unit of pressure is Pascals (Pa) where 1 Pa = 1 N/m .
.
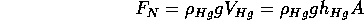

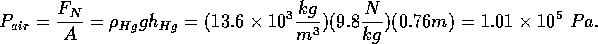

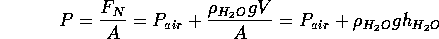

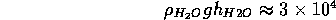





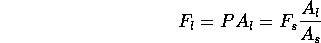

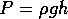 . Therefore the upward force is proportional to
the product of the area and the depth or draught of the boat, that is
the upward force is proportional to the volume of the
water displaced by the boat. The force is equal to
. Therefore the upward force is proportional to
the product of the area and the depth or draught of the boat, that is
the upward force is proportional to the volume of the
water displaced by the boat. The force is equal to 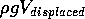 ,
that is it is equal to the ``weight'' of the fluid displaced.
,
that is it is equal to the ``weight'' of the fluid displaced.