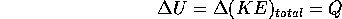
©Timothy E. Chupp, 1995
Processes which involve the movement of heat are called thermodynamic processes. Consider a container of fixed volume with an ideal gas inside. If we add heat to the gas, for example with a flame, and no change of state (i.e solid to liquid or liquid to vapor) the temperature must rise: the heat goes into the total kinetic energy of the molecules of the gas.
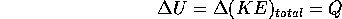
where U is the symbol used for (KE)

and Q is the heat added. This is just the principle of conservation of energy applied to the specific process described above.
Now let's put the piston back into action. If the temperature of the gas increases, the Ideal Gas Law tells us that either the pressure or volume or both must increase. If the gas in the container is allowed to expand and the weight of the plunger does not change, the pressure of the gas does not change and therefore the volume must increase.
For the case of constant pressure, the gas does work on the piston, W=

. The force is the pressure exerted by the gas multiplied by the surface area of the piston:

=

and the change of volume is

=

. Therefore
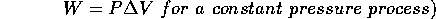
Conservation of energy requires that we must therefore also consider the work done by the gas:

Or in words: the change in the total energy of the gas is the heat added to the gas minus the work done by the gas.
It is useful to show the path of a thermodynamic process on a graph of P vs. V.
For the process of constant pressure expansion, the path is a horizontal line
and the area under the line (or curve) is P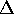 V, the work done by
the gas. In fact the work done by the gas is the area under the curve
on this graph regardless of the nature of the path. To convince yourself
that this is reasonable, break the process up into pieces for which the
pressure is roughly constant (an operation used several times before).
For a given number of moles of an ideal gas, each point on the P vs. V graph
also corresponds to a unique temperature T =
V, the work done by
the gas. In fact the work done by the gas is the area under the curve
on this graph regardless of the nature of the path. To convince yourself
that this is reasonable, break the process up into pieces for which the
pressure is roughly constant (an operation used several times before).
For a given number of moles of an ideal gas, each point on the P vs. V graph
also corresponds to a unique temperature T =  .
.
Figure 1 The P vs V graph. Two kinds of process are particularly useful: isothermal and adiabatic. An isothermal process is one for which the temperature remains constant and therefore
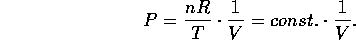
On the P vs. V. graph, this would be a hyperbolic curve resembling that shown. Since the temperature is constant,

U = 0 and
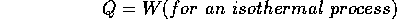
An adiabatic process is one for which no heat is added, i.e. Q= 0. The path of an adiabatic process on the P vs. V graph is shown, but it is not as straight-forward to predict it.
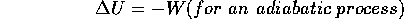
The utility of the isothermal and adiabatic processes arises from the fact (which we'll demonstrate below) that the change of entropy for the process is 0, that is, there is no change in the disorder of the system. Why is this so useful? Because a process for which the entropy does not change can be easily reversed. Consider the system shown below. A reservoir of heat at a constant temperature such as an ice bath at 0C transfers heat to a gas at the same, constant temperature. The work done by the gas is the area under the curve on the P vs. V graph, shown shaded in the figure drawn below. Conservation of energy for this isothermal process requires Q = W. The gas will do work on the piston. The change in entropy of the gas is

and the change in entropy of the reservoir is -

. Thus

S

= 0. Now let the piston do work on the gas until it returns to its initial pressure and volume. The path on the P vs. V graph is the same, only the arrow is reversed and the work done by the gas is of equal magnitude but it is negative as is Q. Again,

S

= 0. For an adiabatic process Q = 0 always and

S = 0 always.
In order to convince yourself that these reversible processes are indeed special, consider the isobaric (constant pressure) transition discussed earlier. As the gas expands with P constant, T =

V will increase. Since the gas is doing work W = P

V and U is increasing, heat must be added: Q =

U + W. This heat comes from a reservoir that must be at a higher temperature than the gas in order for heat to flow in the natural direction. Using the same principles as in the consideration of thermal equilibrium, the total change in entropy is positive. Is this process reversible, that is can the gas be returned to the initial pressure and temperature? In order to do this, work must be done on the gas and it will cool. But if it is still in contact with the reservoir at a higher temperature, heat will not naturally flow from cold to hot. We would need something like a refrigerator to transfer heat from the cooler gas to the warmer reservoir and electrical power for the refrigerator. Think about it: if we allow the gas to expand in a non-reversible way (

S

= 0) we will have to supply extra energy to return it to its initial state!
Truly isothermal or adiabatic process are very hard to realize experimentally. For a gas, the temperature is never completely uniform throughout and therefore even if the container is at a specified temperature, the entire volume of gas is probably not at that temperature. In fact, the only conditions under which a truly isothermal process can occur, i.e. true thermal equilibrium, is at one for which T = 0K. Adiabatic processes require perfect insulation which has not yet been developed, and would also require no molecular motion within the insulation, i.e. T = 0K.
The statements of the last two sections have been consolidated into the three laws of thermodynamics. They are
I. For a system,
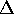 U = Q - W for any process. (This is, of course, just the law of
conservation of energy.)
U = Q - W for any process. (This is, of course, just the law of
conservation of energy.)
II. For any non-reversible process which takes a system from an initial state
(P , V
, V and T
and T ) to a final state (P
) to a final state (P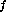 , V
, V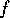 and T
and T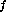 ),
additional energy must be added to return the system to the initial state.
Furthermore, a truly reversible process is possible only at T = 0K.
),
additional energy must be added to return the system to the initial state.
Furthermore, a truly reversible process is possible only at T = 0K.
III. There is a third law which I state with no further discussion because I cannot demonstrate or prove it. It is a form of pessimism: it is not possible to achieve T = 0.
A restatement of the laws might be:
I. You can't win.
II. You can't break even except at absolute zero.
III. You can't reach absolute zero.
A combination of thermodynamic processes that takes a gas from an initial state through other states and returns it to its initial state is called a thermodynamic cycle. Such cycles with working fluids that behave some what like gases (freon is an example) are the basis of engines driven by heat energy and refrigerators or heat pumps which transfer heat from a colder place to a hotter place at the cost of some work. Such a cycle made up of two isothermal processes and two adiabatic processes is shown below. This is a very special and impossible to realize cycle called the Carnot cycle in honor of the thermodynamicist Sadi Carnot. In the cycle shown we note the following properties:
T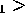 T
T
1. Show that this makes sense, that is show that for the
adiabatic expansion from T to T
to T (where Q=0 and the volume increases)
that the temperature must decrease.
(where Q=0 and the volume increases)
that the temperature must decrease.
During the isothermal expansion at T , the gas does work W
, the gas does work W and
heat Q
and
heat Q = W
= W must be added (
must be added (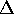 U = 0). Both W
U = 0). Both W and Q
and Q are
positive.
are
positive.
During the isothermal contraction at T , work is done on the gas,
and heat Q
, work is done on the gas,
and heat Q is exhausted, i.e. for this process, the heat added
is -Q
is exhausted, i.e. for this process, the heat added
is -Q and
W
and
W =-Q
=-Q .
.
During the adiabatic expansion and contraction, Q=0.
For the entire cycle, 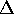 U = 0, i.e. the total change in internal
energy is zero.
U = 0, i.e. the total change in internal
energy is zero.
2. Why is this the case?
Thus W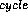 = Q
= Q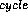 or
W
or
W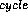 = Q
= Q - Q
- Q .
.
Every process is reversible, thus 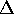 S
S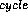 = 0 and
= 0 and
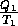 =
= 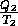 . Therefore Q
. Therefore Q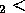 Q
Q .
.
The result of the entire cycle can be summarized: the gas produces useful
work at the expense of heat Q added and heat Q
added and heat Q exhausted as
waste heat: W
exhausted as
waste heat: W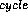 = Q
= Q - Q
- Q . This is of course a common
experience. In an automobile for example, heat is continuously added
by burning fuel but a great deal of heat is wasted as the hot exhaust.
The efficiency of this engine is best defined as the ratio of the useful work
done to the cost in heat energy put in:
. This is of course a common
experience. In an automobile for example, heat is continuously added
by burning fuel but a great deal of heat is wasted as the hot exhaust.
The efficiency of this engine is best defined as the ratio of the useful work
done to the cost in heat energy put in:
 =
= 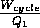 The heat energy Q
The heat energy Q is generally
lost or wasted, though clever schemes abound for
is generally
lost or wasted, though clever schemes abound for
3. Show that for the special Carnot cycle,
 = 1-
= 1-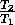
An engine with an efficiency of 1 (100%)
is possible only if T = 0, i.e. at absolute zero, even if it
were possible to create an engine running on the Carnot cycle.
= 0, i.e. at absolute zero, even if it
were possible to create an engine running on the Carnot cycle.
If the arrows on the cycle shown are reversed:
the gas does not do useful work, rather net work is done on the gas:
W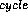 = Q
= Q - Q
- Q which is negative since Q
which is negative since Q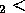 Q
Q .
However the interesting result of this cycle is that heat is removed
from the cooler reservoir at T
.
However the interesting result of this cycle is that heat is removed
from the cooler reservoir at T and heat is exhausted into the
warmer reservoir at T
and heat is exhausted into the
warmer reservoir at T . We have produced a refrigerator: a device
that effects heat flow from a cooler region to a warmer region
at the expense of work. In some applications this device is called
a heat pump and is used to heat a modern house by cooling a
large reservoir, e.g. a pond, and exhausting the heat into the
house.
. We have produced a refrigerator: a device
that effects heat flow from a cooler region to a warmer region
at the expense of work. In some applications this device is called
a heat pump and is used to heat a modern house by cooling a
large reservoir, e.g. a pond, and exhausting the heat into the
house.
Practical heat engines, refrigerators and heat pumps do not work on the Carnot cycle. However the most efficient ones come close to the efficiency given above. As an example, I have shown the cycle used by the steam engine. This is a bit confusing because the process of adding and exhausting heat adds and exhausts gas as well. This twist can be ignored since the mass of the working fluid remains the same.
Physics 125
This document was generated using the LaTeX2HTML translator Version 0.6.4 (Tues Aug 30 1994) Copyright © 1993, 1994, Nikos Drakos, Computer Based Learning Unit, University of Leeds.
The command line arguments were:
latex2html -link 0 -split 0 -no_navigation tseven.tex.
The translation was initiated by Scott D. Dexter on Tue Dec 5 16:32:35 EST 1995