![]()
General information
ASSET (Abatacept Systemic SclErosis Trial) is a clinical research trial designed to assess the efficacy of Abatacept (Orencia®) in patients with diffuse systemic sclerosis. Patients will be randomly assigned to either abatacept or placebo (inert medications) for 12 months, after which all participants will receive abatacept for an additional 6 months. The main purpose of this clinical study is to assess if Abatacept is safe and effective in improving skin fibrosis. The study will also collect skin tissue and blood to understand the pathophysiology of the disease and effect of Abatacept on these markers.
Why diffuse systemic sclerosis?
Systemic sclerosis is a connective tissue disorder affecting close to 300,000 individuals worldwide and 30,000-40,000 in US, characterized by pervasive organ scarring, vascular abnormalities and high mortality. The diffuse scleroderma subset is particularly challenging, as the survival rate is lower, due to earlier and more impactful internal organ damage. While important progress has been made in understanding the disease demographics, clinical history, auto-immune phenotypes and factors implying prognosis, there is no acceptable pharmacological interventions proving curative to date. Among the challenges is lack of understanding the “glitch” (which could be an abnormal cell, or signal pathway) in the immune system function that triggers an absolute and concomitant dysfunction of the inflammatory pathways, vascular function and also the rapid fibrosis.
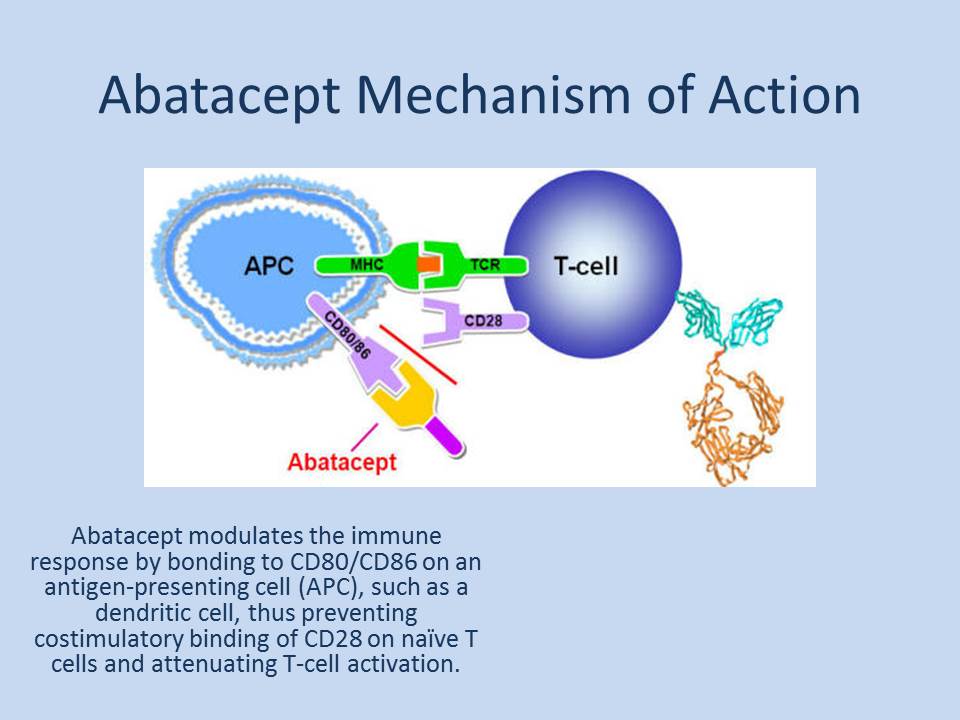
Why Abatacept?
Abatacept is a fusion protein which consists of human CTLA-4 linked to a modified portion of the Fc portion of the human IgG1; it is the first co-stimulation modulator that inhibits T helper cells activation by concomitantly binding to CD80 and CD86 with higher affinity than CD28 (B-cells). This agent was initially approved by the FDA for treatment of rheumatoid arthritis in 2005 under the name of Orencia®, and it was the first to prove the important role of the T cells in the pathophysiology of rheumatoid arthritis. A pilot study by colleagues at Stanford University showed that Abatacept was safe and current study is assessing the effectiveness in a larger trial.
Protocol Synopsis
Protocol Title:
A phase 2 study to evaluate subcutaneous abatacept vs. placebo in diffuse cutaneous systemic sclerosis – a double-blind, placebo-controlled, randomized controlled trial
Sponsor:
This is an Investigator-Initiated study and Dinesh Khanna will be the Sponsor. Bristol Myers Squibb will be supplying the drug/placebo and study funding. The mechanistic study is supported by DAIT, NIAID, and NIH as part of the Clinical ACE grant to UM
Site Numbers:
35 sites in the US, Canada and Europe
Research Hypothesis:
SC abatacept is safe and shows evidence of efficacy (improvement in modified Rodnan score [mRSS]) in patients with diffuse cutaneous systemic sclerosis (dcScc) compared to matching placebo.
Study Schema:
125 mg SC abatacept vs SC placebo administered weekly for 12 months with a 6 month open-label extension.
Study Design:
Double-blind randomized placebo-controlled trial. Subjects will be randomized 1:1 to abatacept or placebo within stratum defined by dcSSc disease duration (≤ 18 months vs.> 18 to ≤ 36 months).
Follow-Up:
12 month double-blind follow-up, followed by 6 month open-label follow-up
Correlative Studies(PK/PD, etc.):
Skin and blood biomarkers
Abbreviated Inclusion Criteria:
1.Diagnosis of SSc, as defined using the 2013 American College of Rheumatology/ European Union League Against Rheumatism classification of SSc
2.dcSSc as defined by LeRoy and Medsger
3.Disease duration of ≤ 36 months (defined as time from the first non−Raynaud phenomenon manifestation)
For disease duration of ≤ 18 months
*10 and ≤ 25 mRSS units at the screening visit
For disease duration of >18-36 months
*≥ 15 and ≤ 45 mRSS units at the screening visit and one of the following:
**Increase ≥ 3 in mRSS units compared with the last visit within previous 1–6 months
**Involvement of one new body area with ≥ 2 mRSS units compared with the last visit within the previous 1–6 months
**Involvement of two new body areas with ≥ 1 mRSS units compared with the last visit within the previous 1–6 months
**Presence of 1 or more Tendon Friction Rub
4. Age ≥ 18 years at the screening visit.
Abbreviated Exclusion Criteria:
1. Rheumatic disease other than dcSSc; it is acceptable to include patients with fibromyalgia and scleroderma-associated myopathy
2. lcSSc or sine scleroderma at the screening visit
3. Major surgery (including joint surgery) within 8 weeks prior to screening visit
4. Infected ulcer prior to randomization
5. Treatment with any investigational agent within ≤ 4 weeks (or 5 half-lives of the investigational drug, whichever is longer) of the baseline visit
6. Severe (MRSS 3+) skin on the inner aspects of thighs, upper arms, and abdomen
7. Previous treatment with cell-depleting therapies, including investigational agents, including but not limited to, CAMPATH, anti-CD4, anti-CD5, anti-CD3, anti-CD19, and ABA
8. Pulmonary disease with FVC ≤ 50% of predicted, or DLCO (uncorrected for hemoglobin ) ≤ 40% of predicted at the screening visit
9. Patients with a history of anaphylaxis to abatacept