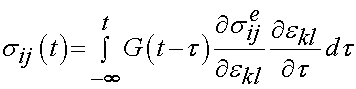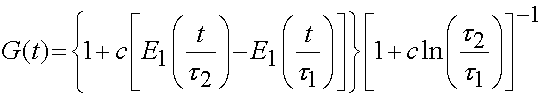BME 456: Biosolid Mechanics: Modeling and Applications
Section 0: Biosolid Mechanics: Modeling and Applications - An Overview
I. Overview
This is a modified class that will focus on modeling and applications of biosolid mechanics to analyze and characterize biological tissue mechanics. The goal of the course is to understand the three most commonly used constitutive models for biological tissues, namely linear/nonlinear elasticity, viscoelasticity, and poroelasticity/biphasic theory, are constructed, how to determine constants for these models using experimental data, and how to use these constitutive models in finite element analysis of biological tissues. It is actually a follow on of the newly developed course BME 332. However, recognizing that a majority of students will NOT have had BME 332, and that a foundation of continuum mechanics is essential to this course, the first few weeks will be spent reviewing continuum mechanics concepts including index notation, stress, deformation, and constitutive models. From then on we will proceed into how one actually determines constitutive model coefficients using experiments and optimization, and see how to use these constitutive equations for finite element analysis. We will also preview how constitutive modeling is used to characterize specific tissue mechanics in relation to application areas of cardiovascular mechanics, orthopaedic soft tissue mechanics, and other soft tissue mechanics. We will also briefly cover modeling of tissue adaptation to mechanical stimulus.
The overall goal of the class is that students understand the fundamentals of continuum mechanics, understand how to develop and utilize the common biological constitutive models of linear/nonlinear elasticity, quasilinear elasticity, and poroelasticity/biphasic theory, and understand how to use finite element methods for stress analysis of biological tissues. Specifically, students should be able to:
1. Understand and be able to use index notation
2. Understand the concept of stress, deformation and strain
3. Understand the concepts and purpose of a constitutive model
4. Understand linear/nonlinear elastic, quasilinear viscoelasticity, and poroelasticity/biphasic constitutive models, including the use of numerical optimization
methods to fit constitutive models to experimental data
5. Learn how constitutive models are applied to model different tissues, including cardiovascular, musculoskeletal and other tissues
6. Understand the concept of finite element modeling and how to create finite element models of tissues
7. Be able to perform a modeling study and communicate results both in writing and orally
II. General Motivation
The general motivation for this class is to provide you with a foundation in continuum mechanics and the methods used by biomechanics researchers used to model biological tissues. Most of the applications will involve soft tissues, but since linear elasticity is one of the most commonly used constitutive theories used to model bone, material covered in this course will be applicable . Of course, one may fairly ask: "Why should I study Biosolid Mechanics? What is it used for?". The applications of biosolid mechanics to clinical medicine are many, although in some ways, these applications are just beginning. For example, clinical applications are as varied as studying how pharmaceuticals used to treat osteoporosis affect bone strength to how diabetes may affect the compliance of arterial walls. Another motivation for studying biosolid mechanics comes from a more basic scientific perspective, namely, many tissues within the body must perform a load bearing function. The question is "How do tissues carry load?". This is a question that many scientists have asked down through the centuries, from Aristotle (384 - 322 BC), who wrote a treatise on the movement of animals,to Galileo Galilee (1564-1642) who surmised that bones gain bending strength by having an increased diameter with hollow center without increasing weight significantly. A interesting concise history of concepts in biosolid mechanics was written by Dr. Bruce Martin and may be found at the website: http://asb-biomech.org/historybiomech/. Thus, biosolid mechanics covers the range from basic to applied science, with significant applications in medicine. In the rest of this overview, I will present some applications of biosolid mechanics to clinical issues including hypertension, ligament injuries, and low back pain. The purpose of these examples is twofold. First, it is to motivate you as to the importance of biosolid mechanics for critical problems in human medicine. Second, it is to motivate you by the complexity of the models to study continuum solid mechanics as a basis understanding biosolid mechanics models.
III. Hypertension - a motivation for studying blood vessel mechanics and nonlinear elasticity
Hypertension, or high blood pressure, is a significant health problem worldwide. Hypertension is a risk factor in a number of other health problems, including stroke, heart failure, intracranial hemorrhage, and atherosclersosis (Humphrey, 2002). Although it is widely accepted that there is a link between hypertension and blood, the plethora of data can often be confusing. For example, it is known that blood vessels become stiffer with age, and that this increased vascular stiffness will lead to increased blood pressure, heart failure, and heart attack (http://www.sciencedaily.com/releases/2001/09/010906072321.htm). Also, a recent study in the American Journal of Hypertension by Grey et al. (2003), noted that increased small artery stiffness was signficantly correlated with a higher occurence of cardiovascular events. Furthermore, Grey et al. found that diet and family history factors including high cholesterol, high blood pressure and diabetes were associated with an increased artery stiffness. It is also known that when hypertension is induced, that this leads to remodeling of blood vessels (that is, a change in blood vessel structure) which in turn leads to a change in blood vessel mechanical properties, generally increasing this properties. Thus, hypertension and blood vessel stiffness may be a postive feedback loop, with one factor increasing the other and vice versa.
Although measures of pulse velocity are related to arterial compliance, we would like to have a very quantitative measure of arterial stiffness to compare between individuals or disease states. As such, we would like to utilize a nonlinear elasticity constitutive model to characterize changes in blood vessels. We now illustrate the use of such nonlinear constitutive models.
In a recent paper, Schulze-Bauer and Holzapfel (2003, J. Biomechanics), proposed a method to determine nonlinear constitutive models for human arteries from clinical data using a strain energy function. There method was based on two assumptions:
1. The axial stretch and axial force of the artery are independent from internal pressure
2. The ratio of circumferential to axial stress is known for one pressure
The first assumption has been shown for rat, rabbit and dog arteries. The second assumption was taken from rabbit arteries. Then, Schulze-Bauer and Holzapfel proposed a strain energy function of the form:

where W is the strain energy function, Eqq and Ezz are components of the finite strain tensor, and C, Cqq, Cqz, and Czz are constants that are to be determined experimentally. By differentiating the strain energy function with respect to the strain components, we can obtain the 2nd Piola-Kirchoff stress components. This can of course be done analytically by hand, but it can also be done using symbolic manipulation software available in MATLAB or MATHEMATICA. The following script shows how this may be done in MATLAB:
First, we define the symbols for the above strain energy function:
>> syms cqq cqz czz c eqq ezz
Note that the subscript "q" denotes q, since q cannot be typed directly in MATLAB. Next, we define the strain energy function in terms of these constants:
>> w = 0.5*c*(exp(cqq*eqq^2 + 2.*cqz*eqq*ezz + czz*ezz^2) - 1)
w =
1/2*c*(exp(cqq*eqq^2+2*cqz*eqq*ezz+czz*ezz^2)-1)
Finally, we can differentiate the strain energy function to obtain the second Piola-Kirchoff stress tensor components. If we first differentiate the strain energy function with respect to eqq, we obtain the stress component sqq as:

To do this in MATLAB, we simply type:
>> sqq = diff(w,'eqq')
sqq =
1/2*c*(2*cqq*eqq+2*cqz*ezz)*exp(cqq*eqq^2+2*cqz*eqq*ezz+czz*ezz^2)
We obtain the same result as if we had differentiated the strain energy function analytically:

we can do the same procedure to obtain the Szz stress component as:

Again, in MATLAB we type:
>> szz = diff(w,'ezz')
szz =
1/2*c*(2*cqz*eqq+2*czz*ezz)*exp(cqq*eqq^2+2*cqz*eqq*ezz+czz*ezz^2)
or again the same as we could obtain analytically:

The constants to be determined experimentally in a constitutive model reflect the underlying physiologic state of the tissue. As such, we can use constitutive models to construct structure-function relationships for tissues, that can relate the physiology or tissue structure, to its function. Schulze-Bauer and Holzapfel were able to obtain fit the above model to experimentally measured pressure-diameter relationships for a 50 year old patient with normal blood pressure and a 52 year old patient with hypertension. The data was obtained by Stefanadis (1995) using an ultrasonic pressure catheter. By fitting the experimental data, Schulz-Bauer and Holzapfel obtained the following experimental coefficients:
Normal Blood Pressure Patient: C(KPa) = 15.4 ; cqq = 2.13 ; cqz = 1.5 ; czz = 1.1
Hypertensive Patient: C(KPa) = 24.4 ; cqq = 7.35 ; cqz = 4.07 ; czz = 2.44
One immediate question is how well the constitutive model can match experimental results. Schulze-Bauer and Holzapfel compared the experimental pressure versus inner diameter of the artery to that predicted by the constitutive model. The open squares represent the experimental data while the solid line represents the prediction from the constitutive model:

As we can see, the constitutive model matches the experimental data very well, indicating the assumptions made in creating the constitutive model are reasonable. Once the constitutive modeling approach is valid, we can then use it to predict the stress-strain behavior of this artery in both the normal and hypertensive patient. Note, however, that if we wish to predict the mechanical behavior of arteries in other patients, or even different arteries in the same patient, it is highly likely that we will need to measure new coefficients for the constitutive model, although the constitutive model itself may take the same form. Schulze-Bauer and Holzapfel also predicted the stress-strain behavior (in this case, strain is represented as a stretch ratio) for the normal and hypertensive patient and obtained the following:


Normal Patient Hypertensive Patient
As we can see from the graphs, the artery in the hypertensive patient develops significantly more stress than the artery in the normal patient. Specifically, if we at the data for a stretch of 1.4 in both the axial and circumferential direction, we can see that the patient with the normal artery develops a sqq stress of about 150 KPa, while the artery in the hyptensive patient develops a stress of about 3 MPa, an increase of nearly 20 times
IV. Ligament Injuries and Healing - A motivation for studying Viscoelasticity
Medial collateal ligament injuries occur frequently in athletic populations, being most numerous in the 20-34 old population. It has been estimated that 300 acute knee injuries occur per 100,000 people in the US per year, or a total of 900,000 acute knee injuries per year (http://www.emedicine.com/pmr/topic72.htm). It is further estimated that collateral ligament damage is involved in over 25% of these acute injuries, for a total of 225,000 knee injuries invovling the collateral ligament every year in the US. Many of these injuries will heal with conservative treatment, that is no surgery. However, knowing when to begin full activity is still a question, as undoubtedly healing ligament mechanical properties are much different from normal ligament mechanical properties.
Of critical interest in the sports medicine community is how the mechanical properties of healing MCL compare to normal MCL. Even though many MCL injuries heal spontaneously, their mechanical properties may not return to normal in a short period of time. Furthermore, since these ligaments are often subject to a myriad of different strain rates in different sports, it is important to understand differences not only in modulus between healing and normal ligaments, but also in time dependent properties. These property differences can only be determined by modeling the ligament as viscoelastic. In viscoelasticity, a peak stress is reached like in elasticity, but we also have stress relaxation over time. To determine these properties, Abramowitch et al. (Annals of Biomedical Engineering, 2004, vol 32) applied a quasilinear viscoelasticty constitutitive model first proposed by Y.C. Fung in 1972. This model is called "quasi" linear because the material is assumed to be linear in its viscous time dependent reponse, but the deformation and instantaneous stress-strain behavior can be nonlinear. Quasilinear viscoelasticity is a very important constitutive model in biomechanics and has been applied to many different soft tissues in order to characterize their time dependent and nonlinear behavior.
The basic stress history form of the quasilinear theory used by Abramowitch et al. is shown below:
In this case, sij is the stress as a function of time, G is the reduced relaxation function, 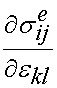 is the instantaneous response of the material, and
is the instantaneous response of the material, and 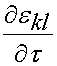 is the strain history. What this equation signifies is that the stress will vary as a function of time, even if the strain is constant over a significant period of time. In fact, if the strain is ramped up and then held constant, the stress will reach a peak and then descrease with time. The instantaneous stress/strain response is modeled using a nonlinear function to account for the nonlinear material and deformation response:
is the strain history. What this equation signifies is that the stress will vary as a function of time, even if the strain is constant over a significant period of time. In fact, if the strain is ramped up and then held constant, the stress will reach a peak and then descrease with time. The instantaneous stress/strain response is modeled using a nonlinear function to account for the nonlinear material and deformation response:
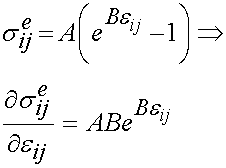
The stress relaxation function is the reduced relaxation function proposed first by Fung:
where E1 denotes the integral:
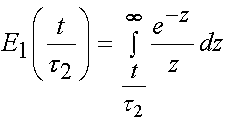
Abramowitch et al. mechanically tested normal goat MCL and MCL that had been ruptured, had the ends reapproximated, and then was allowed to heal for 12 weeks. The ligaments were tested in tension, with the ends still attached to the femur and tibia. This specific type of testing is known as the FMTC for femur-MCL-tibia complex. Abramowitch subjected each ligament to the following strain history:
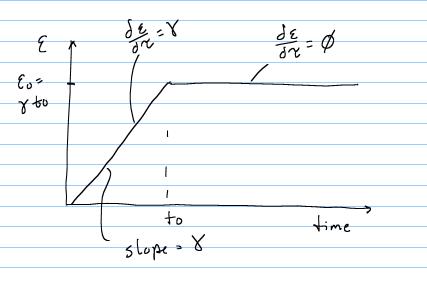
The specific form of the stress history for the above applied strain history can be obtained using the stress history with the reduced relaxation function for t < t0 and t = t0 as:
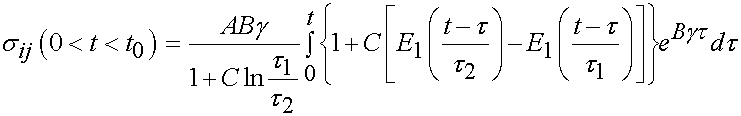
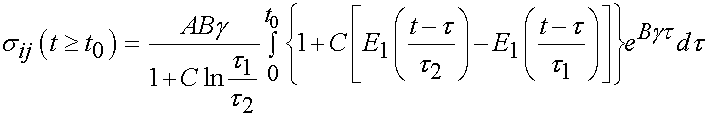
Of course, the challenge is to determine the unknown coefficients of the constitutive model, in this case A, B, C, t1, and t2 that best fit the experimental data. This is best done using an optimization scheme where we try to minimize the error between the experimentally derived stress, and the stress calculated from the constitutive model. This is outlined below:
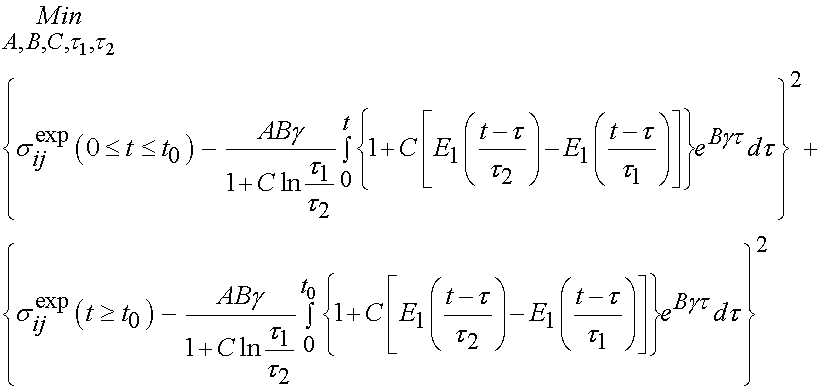
The constitutive model presented above, and the optimization techniques used to fit the model will be covered in class. Doing this, Abramowitch et al. found significant differences between normal and healing ligaments, as shown below:
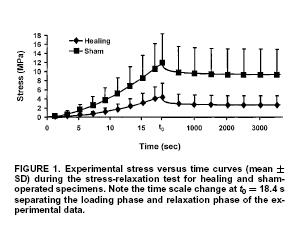
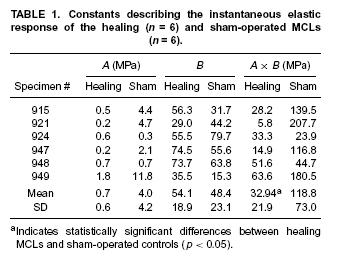
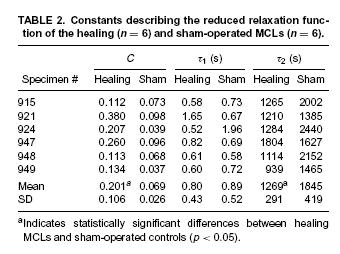
Results indicated that healing ligaments had a lower stiffness, but also had greater stress relaxation and reached an asymptotic level of stress faster after relaxation compared to normal ligaments. This is also demonstrated in the tables giving the constants that were experiementally fit:
V. Low Back Pain and Disc Degeneration- a motivation for studying intervertebral disc mechanics and nonlinear elasticity and poroelasticty
Intervertebral disk degeneration is a leading cause of low back pain that debiltates many people in the US. In fact, low back pain is the leading cause of disability in people under the age of 45. In addition, between $20 and $50 billion is spent every year for treatment cost and disability payments related to back pain (http://yourmedicalsource.com/library/backpain/BAK_whatis.html). Although a very small percentage of the population with back pain end up requiring surgical treatement, there are still over 300,000 spine fusion operations performed in the US every year. Spine fusions are typically performed subsequent to degenerated disk. Degeneration of spinal discs is hypothesized to result from mechanical insult. Adams et al. (Spine, 2000, 25:1625-1636) showed that mechanical damage in the annulus fibrosus would compound and worsen, leading to reduced pressure in the nucleus pulposus. Pictures of the intervertebral disc from the site: http://www.thespinepage.com/anat.htm, by Drs. William Reed, Jr. and Glenn Amundson:


The goal in biosolid mechanics of the intevertbral disc is to develop a constitutive equation that accurately reflects the mechanical behavior of the intervertebral disc. As we will often see is the case with biological tissues, there are different classes of constitutive models that have been developed to describe intervertebral disc behavior. We will briefly touch on examples of two widely used models: nonlinear elasticity and poroelasticity.
A nonlinear anisotropic elastic constitutive model was proposed for intervertebral disk by Klish and Lotz (1999, J. Biomechanics). Since the annulus fibrous contains oriented collagen fibrils. Klisch and Lotz proposed a strain energy function that included terms that accounted for the fibril orientation. Essentially, the collagen fibrils constitute a "net" that helps constrain the fluid filled nucleus pulposus in the center. Klisch and Lotz idealized this fiber net using the following geometric model:

where E1, E2, and E3 are unit vectors in the radial, circumferential, and axial directions respectively. The angle f defines the angle between the two vectors describing the fibril direction with the axial direction. Thus, the vectors a0 and b0 are defined as:

Based on the idea that the fibril orientation will produce a locally orthotropic material, a strain energy function can be in terms of seven invariants:

The In quantities in the strain energy function are invariants of the right Cauchy deformation tensor, and are defined as:

The first general model proposed by Klisch and Lotz had a strain energy function of the form:

where n and all the b terms are coefficients that must be determined by experiment. Since the above model was very senstive during the curve fitting process, Klisch and Lotz later chose a second strain energy function, given below:

where again the a terms are coefficients to be determined by experiment. To determine the experimental coefficients, Klisch and Lotz fit both strain energy functions to experimental data from Iatridis et al. (1998). Iatridis performed the following mechanical tests of intervertebral body tissue:

By curve fitting data from the second model, Klisch and Lotz could show good agreement between their constitutive model and experimental data for the normal vertebral body from Iatridis et al. (1998) as shown below, where the circles are the model results and the solid lines represent the mean behavior and +/- 1 standard deviation:

This showed that the nonlinear elastic model could fit the equilibrium behavior of the intervertebral disk. However, in reality, the intervertebral disk, like many biological tissues, is a combination of a solid and fluid. Therefore, load bearing for time dependent loads is in reality shared between the fluid component and the solid compoent. In fact, if the tissue is permeable, then the fluid flows out more easily and there is a quicker transition between solid and fluid load sharing. In fact, in the original article by Iatridis et al. (1998), the intervertebral disk was modeled using poroelasticity, whether is a mechanics theory that accounts for fluid/solid coupled behavior. In this case, constitutive equations are developed for both the fluid and solid phases. The fluid is assumed to be incompressible with the following constitutive equation:

The solid phase stress is:
![]()
where the last term represents the elastic stress in the solid component. This was represented by Iatridis using the following constitutive model:

where here, Ha is an equilibrium modulus, the stiffness of the tissue after all the fluid has flowed out, l is a stretch ratio, and b is a constant to be determined experimentally. In addition, there is a drag force that couples the fluid and solid behavior, due to the fluid flowing out of the solid. This is given by:

K is the drag coefficient. This is related to the inverse of the permeability by:

where the small "k" is the permeability. Thus, we see that the smaller the permeability, the higher the drag force between fluid and solid. Finally, the permeability depends on the strain and may be written as:

where k0 and M are constants to be fit with experiments from tissues. Iatridis compared the constitutive model coefficients Ha, b, and k0 for normal human cadaver intervertebral disks and degenerated intervertebral disks. Interestingly, they found that the degenerated disks exhibited more linear behavior, (smaller value of b), but were in general stiffer (higher value of Ha). This is reflected in the graph below:

However, Iatridis et al. found no significant difference between the permeability of normal and degenerated disks. Note that both of these results could have been determined with either the Klisch and Lotz constitutive model, or the Iatridis constitutive model. Either way, however, the use of the constitutive model allowed quantification of how a tissues morphological state (normal vs. degenerated) affected its function.
V. Summary
Understand all this? Any of this? None of this? Don't worry! I don't expect you to understand any of this at this point. That is the purpose of the class! By the end of the class, if you flip back to this section, you should be able to understand all of this! The point of this section is both to motivate you and forewarn you. To motivate you, I have tried to pick examples that show biosolid mechanics has important applications in human health. To forewarn you, I have chosen examples that demonstrate the difficulties of studying biosolid mechanics, namely that it can be very complex. That is why we will spend nearly half the term studying the continuum mechanics basis needed to understand the models in biosolid mechanics. This includes index notation, kinematics, stress/force balance and constitutive models
V. References, or where is the book?
All the material for the course will be provided in these web-based notes. The question may arise, why isn't there a textbook? For one thing, we will need to spend a significant amount of time studying continuum mechanics. Therefore, we could use a continuum mechanics book for the class, of which I have borrowed material from two very good ones, one old and one new:
1. Introduction to the Mechanics of a Continous Medium, Lawrence Malvern, 1969
2. Nonlinear Solid Mechanics: A Continuum Approach for Engineering, Gerhard Holzapfel, Wiley, 2002
However, although continuum mechanics books will provide us with the fundamentals, they do not provide the motivation nor examples for studying tissue mechanics. There are some very good biosolid/tissue mechanics books out there, and probably the one that comes closes to the thrust of this class is the book by Fung:
1. Biomechanics: Mechanical Properties of Living Tissues, Y.C. Fung,
Since I wanted to concentrate on more recent work in biomechanics, Fung's book wouldn't do since it was published in 1993, and is now nearly 12 years old. A very good book that I will also draw from for cardiovascular mechanics is:
1. Cardiovascular Mechanics: cells, tissues, and organs, J.D. Humphrey
Again, this is an excellent text, but with a focus on cardiovascular mechanics. Much of the class will be drawn from research papers, some over 25 years old but still extremely relevant, and some just recently published in 2005. If possible, I will upload copies of these papers to the BME 456 CTools site.