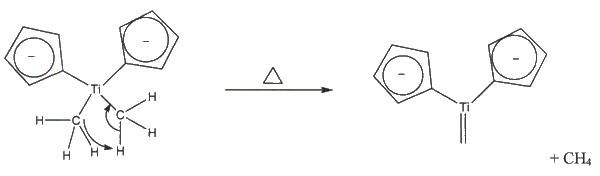|
Petasis
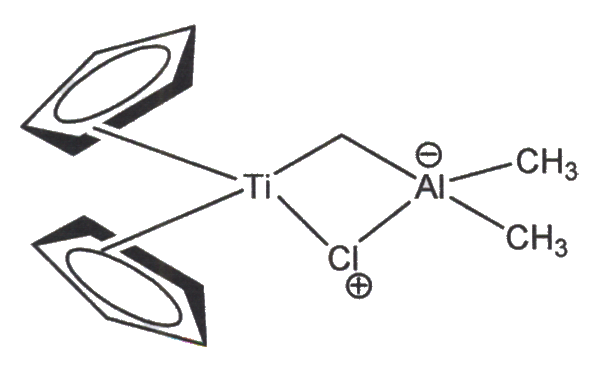
Olefination V. Leading Question Provide a brief lesson to your peers about the Tebbe and Petasis Olefination reactions. What is olefination? Here is the general Theme: Olefination: Invovles a carbonyl group that is transformed into a carbon-carbon double bond (an olefin, or alkene) via a four membered ring intermediate. Many organometallic* reagents can be used, but here is some focus on the Petasis and Tebbe olefinations. *Organometallic compound: characterized by the presence of one or more metal-carbon bonds, in which the carbon involved would, apart form the metal-carbon bond, would be considered an organic compound. Tebbe Reagent A red solid that is pyrophoric (ignites spontaneously in air) and typically used under nitrogen or argon. The Ti and Al bonds are bridged by both CH2 and chloride ligands (an atom, ion, or function that donates its electrons through a coordinate covalent bond, in which only of of the atoms involved in the bond is contributing electrons to the bond) to one or more central atoms or iions, usually metals; an array of such ligands around a center is termed a complex. Tebbe reagent: Cp2TiCh2ClAl(CH3)2
note: Cp aromatic five-membered ring
pi electrons shared by entire structure Petasis Reagent Preparation of Petasis Reagent:
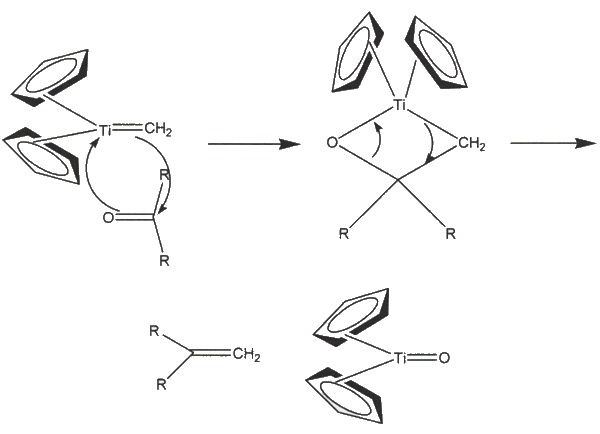
Cp2TiCh2 is what actually does the olefination, and it is known as the Schrock carbene. The preparation of the Hrock carbene gives of methane gas. Here is how an olefination with the Petasis Reagent works:
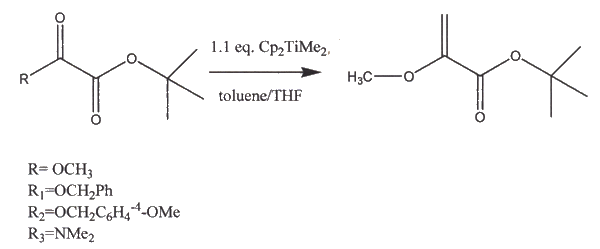
Application: Microwave-assisted, regioselective, petasis Olefination of unsymmetrical oxalates. Formation of pyruvate-based enol ethers and enamines The petasis reagent olefinates ketones, aldehydes, and amides into an alkene via the Schrock carbene efficiently. It does not work well with esters and thioesters. Cook and Fleming prove that even when an excess of the Petasis reagent is use, the non acid sensitive group is chemoselectively favored.
The reaction proceede slwoly when R was used. After 24 hours at 65°C, only there was only a 43% yield of the product. The reaction was driven to completion after the temperature was elevated. However, under microwave conditions, the reaction was driven to completion in only thrity minutes. When the Petasis reagent was subjected to less reactive substrates (R1, R2, R3), reactivity and reaction rate increased when under microwave conditions. In order to drive the reaction all the way to completion, 3 equivalents of the Petasis reagent were used. Even with the excess reagent, only one carbonyl was olefinated (the lessa cid sensitive group, as shown above). Another benefit of the microwave assisted reaction is that a cleaner reaction mixture was obtained. References: 1. Cook, M.J.; Fleming, E.I. Tetrahedron Lett. 2005, 46, 297-300.
2. Morency, L.; Barriaul, L. J. Org. Chem. 2005, 70,
8841-8853.
|