

Why the author cited (16):
(16) Barton, D. H. R.; O'Brien, R. E.; Sternhell, S. J. Chem. Soc. 1962, 470- 476.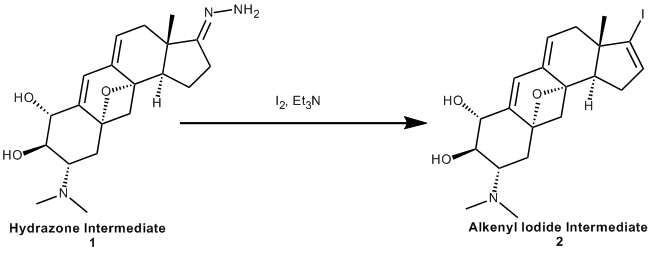
Our reaction from the paper "Synthesis of (+)-cortistatin A" forms the cortistatinone (8) molecule. In the next step of our paper the ketone functional group in cortistatinone reacts with hydrazine (N2H4) to form a hydrazone group (1). This hydrazone then reacts with iodine and triethylamine using a procedure outlined in reference 16. This reference outlines the procedure and mechanism of forming an alkenyl iodide functional group (2) from the hydrazone without affecting other functional groups (such as alcohols). This alkenyl iodide is used in the subsequent Stille coupling step, which is one step away from forming (+)-cortistatin, the final product of our paper.
Three papers that cited reference 16:
Barton, D. H. R.; Bashiardes, G; Fourrey, J. L. Tetrahedron 1988, 44, 147-162.
Cited reference 16 to explain the creation of diiodides from a hydrazone formed from an aldehyde reacting with iodine and triethylamine.
Nicolaou K. C.; Mathison C. J. N.; Montagnon T. J. Am. Chem. Soc. 2004, 126, 5192-5201.
Cited reference 16 to show another way to perform the same dimerization that they performed, the use of iodine on a hydrazone.
Barton, D. H. R.; Bashiardes, G; Fourrey J. L. Tetrahedron Lett. 1983, 24, 1605-1608.
Cited reference 16 because the same reaction is being carried out in this paper except instead of using a hydrazone formed from an aldehyde, as in 16, this paper uses a hydrazone formed from a ketone to form vinyl iodide.
Citations
