

Glossary Of Terms with structures:
- Azeotrope : An azeotrope is a mixture of two or more liquids that its composition cannot be changed by simple distillation.
The reason this occurs is because when the azeotrope is boiled, the same ratio of liquids is evaporated
- DCM: Dichloromethane is a highly used solvent that is capable of dissolving many organic solutes.
- In Vacuo: The Latin term in vacuo is also used to describe an object as being in what would otherwise be a vacuum
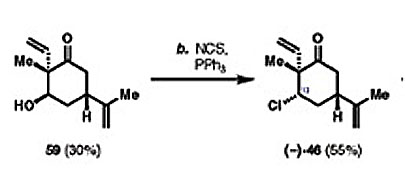
-N-chlorosuccinimide (NCS): a mild oxidant (oxidating reagent that gains electrons through an oxidation reaction LEO goes GER) that is used in chlorination reactions.
(Chlorination being the process of adding chlorine to water)
| NCS | 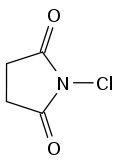 |
| THF |  |
 |
The envious scientist is jealous of your knowledge of organic chemistry.
Run back to the Frontpage if you want to avoid a nitroglycerine explosion.
Home | Mechanism | NMR Citation | Leading Question | About Us
Eugene Lo, Mike VanHall, Anna Shatsman