
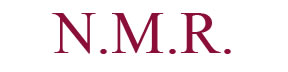

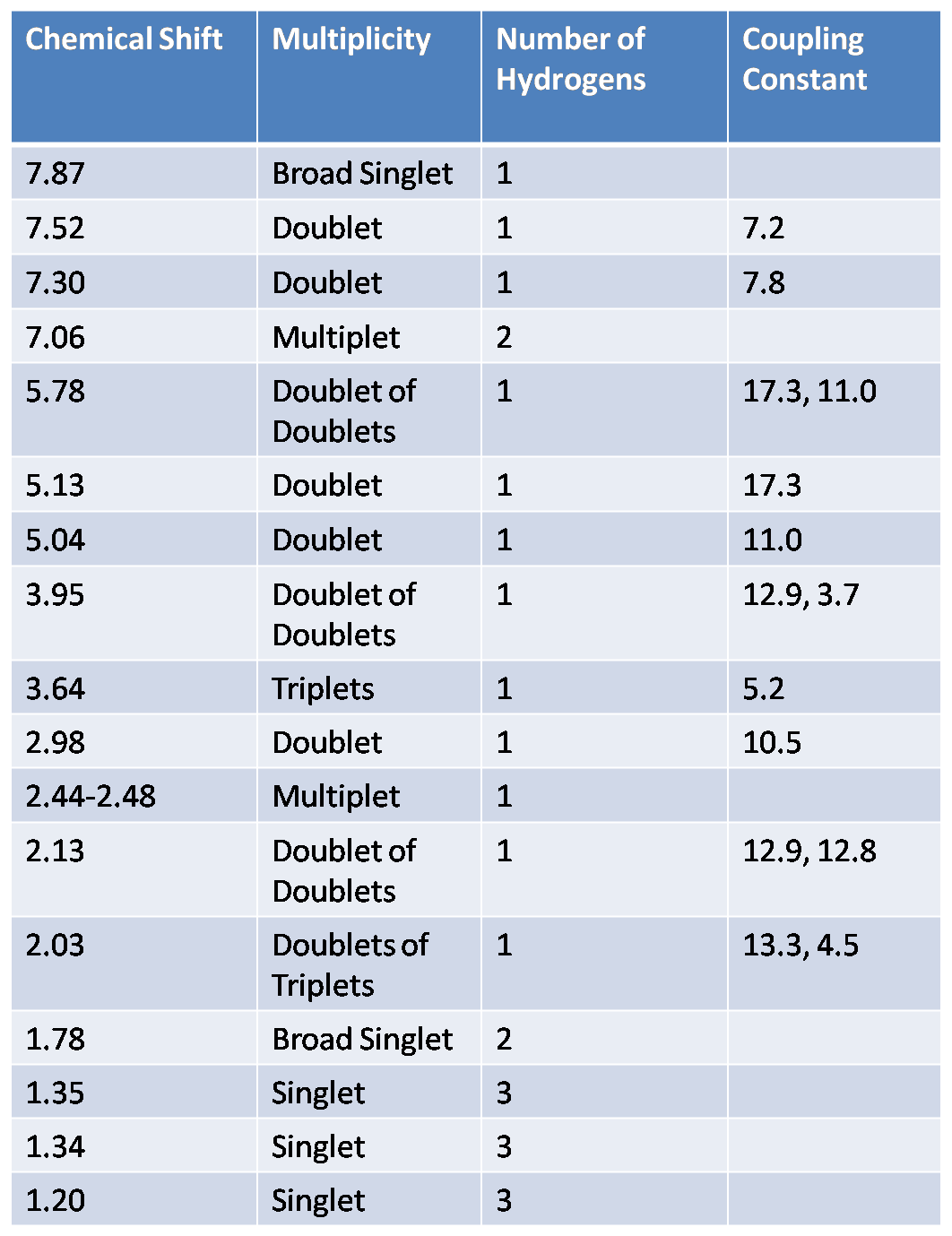
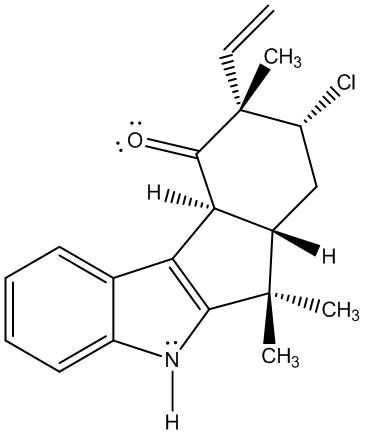
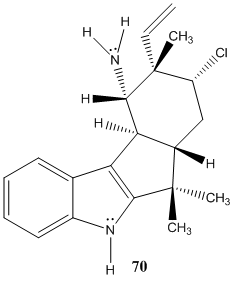
 s
s
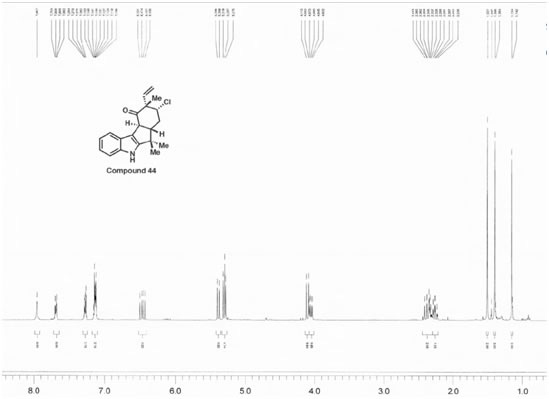
44
364, 203
Molecule 44 Explanation
Molecule 44 contains three methyl groups. None of these methyl groups couple with another molecules, and as a result, each are represented on the H NMR spectra as singlets with high peak intensities (displaying that each peak accounts to three hydrogens). Two are bonded to the same carbon, and the other methyl group is located near an alkene. Three singlets appeared on the H NMR spectra: 1.51 ppm, 1.40 ppm, and 1.15 ppm. The singlet of the methyl near the alkene is believed to correspond to the peak located at 1.15 ppm. A methyl is generally found near 1 ppm, and the presence of the alkene does not shift the methyl group down field; however, the chlorine atom that is two bonds away deshields the methyl group (making it more down field shifted). The other two methyl groups correspond to the 1.51 and 1.40 ppm values. These two groups are near a very electronegative nitrogen atom which results in their down field shifts. There are more objects located on the bottom-half of the molecule, so the methyl group on the dash is believed to correspond to the peak at 1.40 ppm. Moreover, the multiple substituents on the bottom portion of the molecule result in an additive effect that does not allow the molecule to as down field shifted as would the methyl group on a wedge. The methyl group on the wedge is near the nitrogen atom and as a result it is sufficiently shifted down field; as a result it is believed to be the singlet at 1.51 ppm. Next, there is a carbon located one bond away from the carbon with the chlorine atom, and this carbon atom has two hydrogens attached to itself. These hydrogens are not chemically equivalent, and they couple with two hydrogens that are not chemically equivalent as well. There is one non-chemically equivalent hydrogen bonded to the carbon bonded to a chlorine atom, and another is a nearby hydrogen located on a dash. Moreover, coupling with two non-chemically equivalent hydrogens results in a doublet of doublets. However, there is another doublet of doublets located on the H NMR. Furthermore, that peak is believed to correspond to the hydrogen located on the alkene, which is also bonded to a hydrogen on a dash. Through coupling in an alkene system, this hydrogen is a doublet of doublets. One of the hydrogens located near the chlorine atom would be located at 4.05 ppm, significantly high for a CH2 group because it is located two bonds away from a chlorine atom (the atom’s nucleus has been deshielded). Since the spectral data was rather impure, only one J-value was found. This coupling constant was at 11.2 Hz. The hydrogen being examined is believed to be on a dash and is axial, and is believed to be coupling with the hydrogen that is located one carbon away and is on a dash (believed to be axial as well). Moreover, these two hydrogens are participating in an axial-axial interaction and therefore have a rather high J-value (usually between 10 and 12 Hz). The dihedral angle must be around 180 degrees, and according to the Karplus Curve, such a dihedral angle results in a large coupling constant. The remaining hydrogen on that same carbon would logically be a doublet of doublets as well, but due to the spectral data not being entirely pure; furthermore, three doublet of doublets would be expected and the data does not reflect this necessity. Thus, the other doublet of doublets corresponds to the hydrogen on the alkene (6.47 ppm). Hydrogens attached to alkenes are generally located around 6.5 and 4.5 ppm. So this increases the likelihood that the peak at 6.47 ppm corresponds to the alkene hydrogen. There are two J values for this hydrogen (17.6 Hz and 10.8 Hz); moreover, the hydrogen couples with the cis hydrogen (at the other end of the alkene and on the same side) resulting in the 10.8 Hz coupling constant. The 17.6 Hz coupling constant is characteristic of alkene hydrogen coupling with a trans hydrogen (at the other end of the alkene and on the opposite side). The next two hydrogens of interest are the other hydrogens attached to the alkene. One hydrogen is cis to the hydrogen at the opposite end of the double bond, and the other hydrogen is trans to this hydrogen. Trans hydrogens in an alkene have higher chemical shift values (more down field shifted) than cis hydrogens in an alkene. Yet, the ppm values had a difference of only 0.8 ppm, so the J-values were observed. The J-value at 5.38 ppm was 10.8 Hz, and the J-value at 5.30 ppm was 17.6 Hz. These results are counter-intuitive because cis hydrogens typically have J-values at around 8 and 10 Hz and should be less down field shifted than a trans hydrogen, but the hydrogen at 5.30 ppm is believed to be trans because its J-value is so large. These two hydrogens are both doublets because they couple with the hydrogen atom at the other end of the alkene. The broad singlet at 7.96 ppm is undeniably the hydrogen attached to the nitrogen molecule. One is behooved to note that hydrogens attached to nitrogens and oxygens are generally broad singlets, and are very down field shifted due to the fact that they are bonded to very electronegative atoms. The last four peaks were all multiplets. In the aromatic ring at the very left of 44, there are two chemically equivalent hydrogen atoms bonded to a double bond. The other two hydrogens in this ring are not chemically equivalent and are closer to the electronegative nitrogen atom than the two aforementioned hydrogens. Thus, the chemically equivalent hydrogens correspond to the peak at 7.11-7.15 ppm. The hydrogen located at the northern region of the ring is four bonds away from the nitrogen atom and the hydrogen at the southern most region of the ring is three bonds away from the nitrogen atom. The inductive effect has a grander effect on the southern most hydrogen because they are less bonds away from one another than the other northern hydrogen. Thus, the southern hydrogen has a ppm of 7.70 to 7.68. Leaving the other hydrogen with a ppm of 7.28-7.26 ppm. The last multiplet is rather unexplainable. There are three hydrogen atoms remaining and are all a part of a non-aromatic ring. They would be expected to be a doublet, a doublet of doublets of doublets, and a doublet of doublets. The coupling of these hydrogens must be so strong and impure that a multiplet has been seen in the spectral data.
Molecule 70 Explanation
Molecule 70 contains three methyl groups. None of these methyl groups couple with another molecules, and as a result, each are represented on the H NMR spectra as singlets with high peak intensities (displaying that each peak accounts to three hydrogens). Two are bonded to the same carbon, and the other methyl group is located near an alkene. Three singlets appeared on the H NMR spectra: 1.35 ppm, 1.34 ppm, and 1.20 ppm. The singlet of the methyl near the alkene is believed to correspond to the peak located at 1.20 ppm. A methyl is generally found near 1 ppm, and the presence of the alkene does not shift the methyl group down field; however, the chlorine atom that is two bonds away deshields the methyl group (making it more down field shifted). The other two methyl groups correspond to the 1.35 and 1.34 ppm values. These two groups are near a very electronegative nitrogen atom which results in their down field shifts. There are more objects located on the bottom-half of the molecule, so the methyl group on the dash is believed to correspond to the peak at 1.34 ppm. Moreover, the multiple substituents on the bottom portion of the molecule result in an additive effect that does not allow the molecule to as down field shifted as would the methyl group on a wedge. The methyl group on the wedge is near the nitrogen atom and as a result it is sufficiently shifted down field; as a result it is believed to be the singlet at 1.35 ppm. The next peak is a broad singlet at 1.78 ppm. This peak corresponds to the two hydrogens bonded to the nitrogen atom. One is behooved to note that hydrogens attached to nitrogens and oxygens are generally broad singlets, and are generally very down field shifted due to the fact that they are bonded to very electronegative atoms. Next, there is a carbon located one bond away from the carbon with the chlorine atom, and this carbon atom has two hydrogens attached to itself. The doublet of triplets at 2.03 ppm represents one of these hydrogens. This hydrogen couples with the chemically non-equivalent hydrogen bonded to the same carbon, as well as two other chemically non-equivalent hydrogens. The coupling constants are 13.3 Hz and 4.5 Hz. The 13.3 Hz corresponds to the CH2 coupling, which is characteristically between 10 and 15 Hz. The newly added hydrogen (which is on a wedge) appears as a doublet at 2.98 Hz and is believe to be axial since the hydrogen it couples with is on a dash, and their coupling constant is 10.5 Hz. Axial –axial couplings have large J-values around 10 and 12 Hz. The dihedral angle must be around 180 degrees, and according to the Karplus Curve, such a dihedral angle results in a large coupling constant. The doublet of doublets at 5.78 ppm corresponds to the hydrogen on the alkene. Hydrogens attached to alkenes are generally located around 6.5 and 4.5 ppm. So this increases the likelihood that the peak at 5.78 ppm corresponds to the alkene hydrogen. There are two J values for this hydrogen (17.3 Hz and 11 Hz); moreover, the hydrogen couples with the cis hydrogen (at the other end of the alkene and on the same side) resulting in the 11 Hz coupling constant. The 17.3 Hz coupling constant is characteristic of alkene hydrogen coupling with a trans hydrogen (at the other end of the alkene and on the opposite side). The next two hydrogens of interest are the other hydrogens attached to the alkene. One hydrogen is cis to the hydrogen at the opposite end of the double bond, and the other hydrogen is trans to this hydrogen. Trans hydrogens in an alkene have higher chemical shift values (more down field shifted) than cis hydrogens in an alkene. Yet, the ppm values had a difference of only 0.8 ppm, so the J-values were observed. The J-value at 5.04 ppm was 11 Hz, and the J-value at 5.13 ppm was 17.3 Hz. These results are counter-intuitive because cis hydrogens typically have J-values at around 8 and 10 Hz and should be less down field shifted than a trans hydrogen, but the hydrogen at 5.13 ppm is believed to be trans because its J-value is so large. These two hydrogens are both doublets because they couple with the hydrogen atom at the other end of the alkene. The hydrogen attached to the carbon bearing the chlorine atom is a doublet of doublets at 3.95 ppm. There is an adjacent carbon with two chemically non-equivalent hydrogens, resulting in the doublet of doublets. The coupling constants are 12.9 and 12.8 Hz. The broad singlet at 7.87 ppm is undeniably the hydrogen attached to the nitrogen molecule. The last three peaks were all multiplets. In the aromatic ring at the very left of 70, there are two chemically equivalent hydrogen atoms bonded to a double bond. The other two hydrogens in this ring are not chemically equivalent and are closer to the electronegative nitrogen atom than the two aforementioned hydrogens. Thus, the chemically equivalent hydrogens correspond to the peak at 7.06 ppm. The hydrogen located at the northern region of the ring is four bonds away from the nitrogen atom and the hydrogen at the southern most region of the ring is three bonds away from the nitrogen atom. The inductive effect has a grander effect on the southern most hydrogen because they are less bonds away from one another than the other northern hydrogen. Thus, the southern hydrogen has a ppm of 7.70 to 7.68. Leaving the other hydrogen with a ppm of 7.28-7.26 ppm. The triplet is rather unexplainable since it was not present in 44.
.