Science Citation Index
Articles citing Dehydration of formamides using the Burgess Reagent: a new route to isocyanides
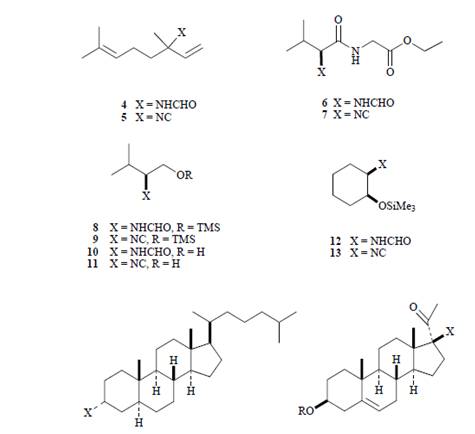
Converting molecule 44 to molecule 70 requires utilizing an isocyanide molecule. A common way to prepare isocyanides is by the dehydration of formamides, however the Burgess reagent was applied to the reaction, resulting in a highly effective reaction to N-formamides. While the Burgess reagent was not utilized to convert molecule 44 to molecule 70, it was used to converted molecule 70 to molecule 72. The Burgess reagent is halide free, and especially effective in dehydrating alcohols. A common reaction set up included adding the drying agent to an amide solution, and adding this to dry methylene chloride. It is recommended that an excess of methylene chloride, be applied because of the reagents sensitivity to moisture. Chromatography was used to isolate and purified most of the newly formed isocyanide product. The following molecules demonstrate the variety of molecules that may be reacted using the Burgess reagent.

1. Passerini Reaction- Amine Deprotection-Acyl Migration Peptide Assembly: Efficient Formal Synthesis of Cyclotheonamide C
Cyclotheonamide is formed via the synthesis of three molecules, with an isonitrile and a hydroxyhomoarginine adding to a pentapeptide, which includes nitrogen bonding similar to the Burgess reagent. Faure, S.; Hjelmgaard, T.; Roche, S.; Aitken, D., Org. Letts. 2009, 11, 1167-1170. 2. N-Arylimidazole synthesis by cross-cycloaddition of isocyanides using a novel catalytic system.
N-arylimidazoles are synthesized from a corresponding N-arylformamide and N-formylglycine, creating a two aromatic ring system connected by a nitrogen carbon bond.
Bonin, M.; Giguere, D.; Roy, R., Tetrahedron, 2007, 63, 4912-4917.
|
3. New application of Burgess reagent in its reaction with epoxides.
The Burgess reagent was successfully utilized to form sulfamidites from their corresponding epoxides, and to form seven-membered rings from aromatic epoxides.
Rinner, U; Adams, D. R.; Santos, M. L; Abboud, K. A.; Hudlicky, T.,
Synlett. 2003, 9, 1247-1252.