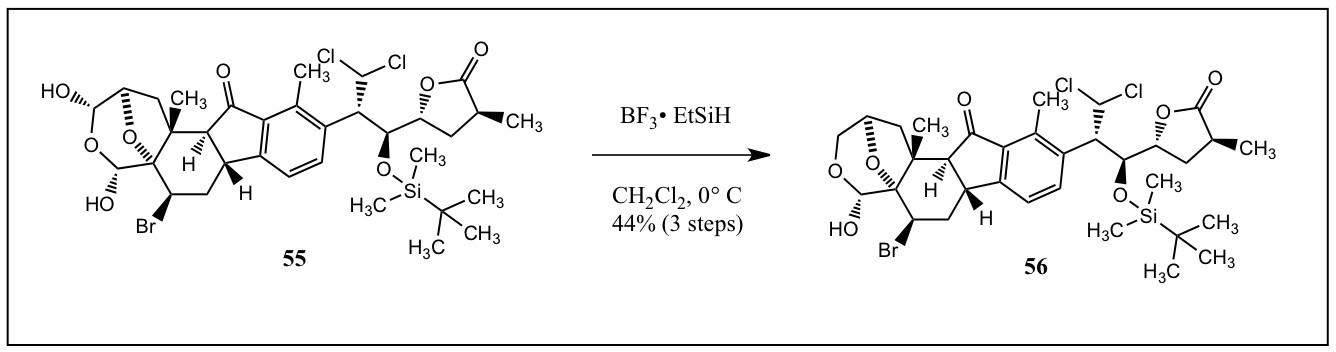
|
56. To a previously obtained solution of 55, borontrifluoride diethyl etherate and triethylsilane in methylene chloride were slowly added at 0 °C. After stirring at same temperature for 2 hours, a saturated aqueous solution of sodium bicarbonate was added. The biphasic mixture was extracted with ethyl acetate, and the organic phase was dried over anhydrous sodium sulfate, filtered, concentrated, and purified by silica gel column chromatography (20% ethyl acetate–hexanes). 1H NMR: δ 7.90 (d, 1H, J=8.0 Hz), 7.34 (d, 1H, J=8.0 Hz), 6.32 (d, 1H, J=10.0 Hz), 5.29 (s, 1H), 4.71 (dd, 1H, J=3.5 Hz, 2.5 Hz), 4.41 (dd, 1H, J=7.9 Hz, 3.2Hz), 4.25 (dd, 1H, J=7.8 Hz), 4.10 (d, 1H, J=11.0 Hz), 3.93 (ddd, 1H, J=8.1 Hz, 7.9 Hz, 3.9 Hz), 3.89 (dd, 1H, J=10.0 Hz, 3.2 Hz), 3.55 (ddd, 1H, J=12.7 Hz, 9.4 Hz, 2.3 Hz), 3.12 (dd, 1H, J=11.0 Hz, 1.0 Hz), 2.75 (ddd, 1H, J=13.7 Hz, 2.5 Hz, 2.3 Hz), 2.72 (s, 3H), 2.73-2.69 (1H, m), 2.70 (d, 1H, J=9.4 Hz), 2.29 (ddd, 1H, J=13.7 Hz, 12.7 Hz, 3.4 Hz), 2.61 (dd, 1H, J=12.4 Hz, 7.87 Hz), 2.06 (dd, 1H, J=12.4 Hz, 1.0 Hz), 1.72 (ddd, 2H, J=13.2 Hz, 8.2 Hz, 8.1 Hz), 1.51 (s, 3H), 1.15 (d, 3H, J=7.3 Hz), 0.95 (s, 9H), 0.28 (s, 3H), 0.15 (s, 3H), 4.00-1.57(s, 1H).
Note: The experimental for this step was extrapolated from that for the synthesis of compound 10 from 29 on page 378 as the exact experimental for 55 to 56 was not given and the step of 29 to 10 uses the same reagents and reaction conditions.
|