Miss Peacock wants to know where all these brilliant people found this information. We found the answers for her and created this web page! |
|
Articles about Macrolactonization: |
|
| Fraunhoffer, K. J.; Prabagaran, N.; Sirois, L. E.; White, M. C. J. Am. Chem. Soc. 2006, 128 (28), 9032-9033. | |
| Trost, B. M.; Chisholm, J. D. Org. Lett. 2002, 4 (21), 3743-3745. | |
Keck cites an article in his paper that is relevant to the chemistry that is discussed on this website. It's citation is shown below. |
|
| Inanaga, J.; Hirata, K.; Saeki, H.; Katsuki, T.; Yamaguchi, M. Bull. Chem. Soc. Jpn. 1979, 52, 1989–1993. | |
Why does Keck cite this article, and what conclusions does it come to about the chemistry of the reaction of Molecule 25 to 26? |
|
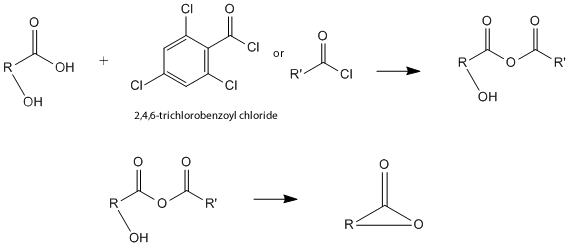
| This article is cited by the Keck article because this article describes the conditions that our reaction takes place under. However, this article that the Keck article cites provides some information about the mechanism of this reaction. Based on this article, the reaction proceeds through the formation of a mixed anhydride. This article describes the many trials and errors of determining the best and most efficient way to prepare acid anhydrides, as well. They found that using acid chlorides, or reacting a carboxylic acid with 2,4,6-trichlorobenzoyl chloride, was the best way to create a high yield of mixed anhydride product, suggesting that this mechanism is the best for preparing the reactant. So, the first step in this reaction forms the mixed anhydride using this reaction and the carbonyl functional group in the molecule. The next crucial step of this mechanism is the attack of the mixed anhydride by the alcohol. This step can also be called alcoholysis. In this lactonization reaction, the alcohol must come from within the molecule because, by definition, lactonization is an intramolecular reaction. This mechanism, through these reactants, produces the final product, the macrolactone, most effieciently and in the highest yield. The simplified version of the mechanism, as described above is shown in the images below. | |
 |
|
Articles that cite the Yamaguchi article and their reasons for this citation: |
|
| Gardinier, K. M.; Leahy J. W. J. Org. Chem. 1997, 62 (21), 7098-7099. | |
This article cites the Yamaguchi article because it uses the same reaction conditions described in the Yamaguchi article to close a ring, producing a macrolide, as a step in synthesis. |
|
| Kigoshi, H.; Ojika, M.; Ishigaki, T.; Suenaga, K.; Mutou, T.; Sakakura, A.; Ogawa, T.; Yamada, K. J. Am. Chem. Soc. 1994, 116 (16), 7443-7444. | |
This article cites the Yamaguchi article because it produces a 24- and 26- membered macrolactone using the methods described in the Yamaguchi article. |
|
| Rowan, S. J.; Hamilton, D. G.; Brady, P. A.; Sanders, J. K. M. J. Am. Chem. Soc. 1997, 119 (10), 2578-2579. | |
|