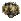
Experimental
According to the experimental section of Grand Master Koreeda's notebook, the procedure seems to be relatively easy compared to the numerous other chemical experiments they have performed. However, there seems to be one fundamental equipment called 'microwave', which the eccentric yet the best Gnome engineers presented to the Grand Master.
The only note on experimental section is the following:
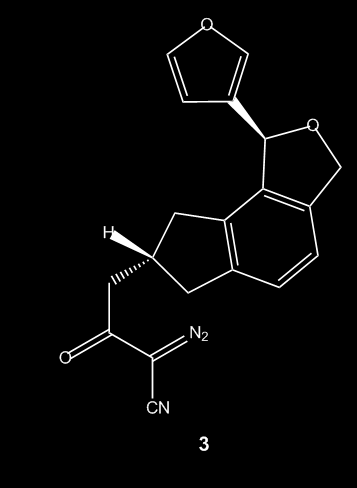
A 20 mL microwave vial was charged with Cu(hfacac)2 (14.9 mg, 0.03 mmol), sealed, and purged with N2. To a flame-dried 25 mL flask containing diazo β-ketonitrile 3 (100 mg, 0.30 mmol, 10:1 mixture diastereomers) was charged DCM (15 mL). The diazo solution was then transferred via syringe into the sealed microwave vial. The vial was placed into the microwave reactor and heated to 120 °C for 1 minute (ramp from 22 °C to 120 °C required 1.5 minutes). TLC analysis confirmed consumption of the starting material, and the solvent was removed in vacuo. The crude residue was purified by flash chromatography (gradient elution, 0 → 60% EtOAc/Hexanes), yielding norcaradiene 15 (60 mg, 65% yield, single diastereomer) as a white crystalline solid.
Though it is not clear how to use the equipment properly, all of his students are cautiously trying to figure out how it works.
Compounds:
Glossary
Microwave reactor:

Flash Chromatography
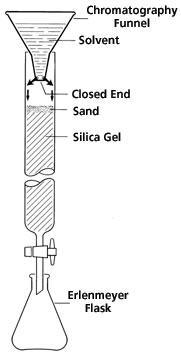
Flash Chromatography is a rapid form of column chromatography, made possible by low pressure. The column is first filled with dry stationary phase powder, followed by the addition of mobile phase, which is flushed through the column until it is completely wet, and from this point is never allowed to run dry. The compounds will be separated as two separate eluents.