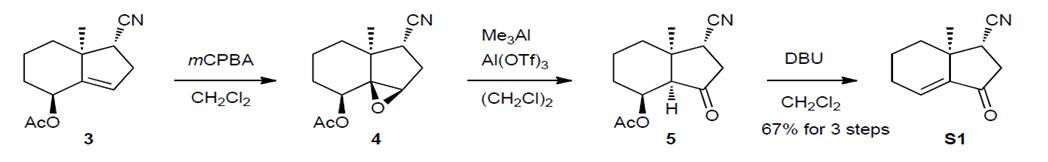
Compound S1: To a solution of acetate 31 (8.77 g, 40.0 mmol) in CH2Cl2 (160 mL) was added m-chloroperbenzoic acid (mCPBA) (75% purity, 18.4 g, 80.0 mmol) at 0 ºC. After being stirred for 2 h, an excess amount of 2-methyl-2-butene was added to reduce mCPBA, and the ice bath was removed. After being stirred for 10 min, a saturated aqueous solution of NaHCO3 was added. The mixture was separated and the aqueous layer was extracted with ether. The combined organic layer was washed with a saturated aqueous solution of NaHCO3 and brine, dried over MgSO4 and concentrated under reduced pressure. The crude epoxide 4 was used for the next step without purification. To a suspension of Al(OTf)3 (37.9 g, 80 mmol) in hexane (20 mL) was added a 2.0 M hexane solution of Me3Al (20 mL, 40 mmol) at 0 °C, and the mixture was stirred at room temperature for 2.5 h. To this was added a solution of the crude epoxide 4 in 1,2-dichloroethane (160 mL), and the reaction mixture was gradually warmed up to 80 ºC over 1 h. After being stirred for 8.5 h, the mixture was cooled to room temperature, and a saturated aqueous solution of NaHCO3 followed by a saturated aqueous solution of potassium sodium tartrate (Rochelle salt) were added carefully. The mixture was separated and the aqueous layer was extracted with ether. The combined organic layer was washed with brine, dried over MgSO4, and concentrated under reduced pressure. The crude ketone 5 was used for the next step without purification. To a solution of the crude ketone 5 in CH2Cl2 (160 mL) was added 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) (6.6 mL, 44.0 mmol) at 0 ºC. After being stirred for 2 h, a saturated aqueous NH4Cl solution was added. The mixture was separated and the aqueous layer was extracted with ether. The combined organic layer was washed with brine, dried over MgSO4, and concentrated under reduced pressure. Purification by silica gel column chromatography (EtOAc/hexane 1:11.5 then 1:7.3) afforded enone S1 (4.7 g, 26.8 mmol, 67% for 3 steps) as a white solid: m.p.: 78–79 ºC; [!]26D (deg cm3 g–1 dm–1)= –327 (c=0.73 g cm–3 in CHCl3); IR (KBr): 2240, 1720, 1650 cm–1; 1H-NMR (500 MHz, CDCl3): " 6.74 (t, J=3.7 Hz, 1H), 2.75–2.60 (m, 3H), 2.42–2.34 (m, 1H), 2.26–2.12 (m, 2H), 1.89–1.72 (m, 2H), 1.36–1.26 (m, 4H involving a singlet at 1.30); 13C-NMR (125 MHz, CDCl3): " 199.95, 141.89, 135.13, 118.84, 40.98, 38.56, 37.01, 34.44, 24.99, 21.63, 17.86; HRMS (EI, m/z): [M+] calcd for C11H13NO, 1750997; found, 175.0999.