Now that you’ve reached the Lollipop Woods, it’s time to inspect the procedure! Move carefully through the candied trees to properly execute each step – otherwise you could run into some sticky situations!
Experimental
Compound S5
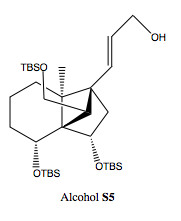
To a solution of ester 11 (3.22 g, 4.93 mmol) in THF (25 mL) was added a 1.02 M hexane solution of DIBAH (14.5 mL, 14.8 mmol) at –78 ºC. After being stirred for 45 min, a saturated aqueous Rochelle salt solution was added. The mixture was separated, and the aqueous layer was extracted with ether. The combined organic layer was washed with brine, dried over MgSO4, and concentrated under reduced pressure. Purification by silica gel column chromatography(EtOAc/hexane 1:13.3) afforded alcohol S5 (3.0 g, 4.91 mmol, 100%) as a colorless oil.
Compound 12
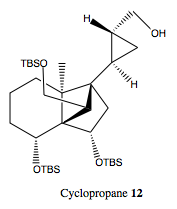
To a mixture of a 1.05 M hexane solution of Et2Zn (11.5 mL, 12.1 mmol) and CH2Cl2 (15 mL) was added diiodomethane (2.0 mL, 24.9 mmol) at 0 ºC. After the reaction mixture was turned into a white suspension, a solution of alcohol S5 (2.44 g, 4.0 mmol) and the dioxaborolane ligand (1.3 g, 4.8 mmol) in CH2Cl2 (25 mL) was added. After being stirred vigorously at room temperature for 3 hours, a saturated aqueous NH4Cl solution was added. The mixture was separated, and the aqueous layer was extracted with ethyl acetate. The combined organic layer was washed with brine, dried over MgSO4, and concentrated under reduced pressure. The residue was dissolved in THF (20 mL), and to this was added a 1 M aqueous NaOH solution (10 mL) and a 30% aqueous H2O2 solution (10 mL) at 0 ºC. After being stirred for 10 min at 0 ºC, the mixture was separated, and the aqueous layer was extracted with ether. The combined organic layer was washed with brine, dried over MgSO4, and concentrated under reduced pressure. Purification by silica gel column chromatography (hexane then EtOAc/hexane 1:19) afforded cyclopropane 12(2.50 g, 4.0 mmol, 100%) as a colorless oil containing a trace amount of inseparable diastereomer.
Glossary
Alcohol S5: The product of the first stage of this step in the reaction, is used to produce cyclopropane 12
Brine: A saturated solution of salt that is used to remove water from a reaction.
Cyclopropane 12: The final product of this step in the reaction. The carboxylic ester has been turned into a primary alcohol and the alkene has been converted into a cyclopropane ring.
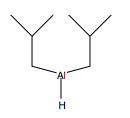
DIBAH: Diisobutylaluminum hydride is a reducing agent similar to LiAlH4 that reduces carbonyl derivatives to alcohols and aldehydes and nitriles to amines.
Diiodomethane: Has the formula CH2I2 and provides the 3rd carbon atom in the cyclopropane ring.
Dioxaborolane Ligand: A reagent used to control the stereochemistry of the cyclopropanation reaction. The ligand will convert alkenes with an attached allylic alcohol into an anti-cyclopropane ring.
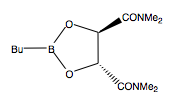
Ester 11: The starting material used in this step of the reaction. Contains the carboxylic ester and alkene needed for both parts of this step.
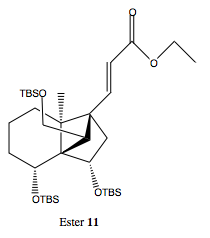
Et2Zn: Diethylzinc is used in combination with diiodomethane as a Simmons-Smith reagent in the second part of this step of the reaction.
Rochelle Salt Solution: Also known as potassium sodium tartrate, Rochelle’s salt is used to break up aluminum reagent emulsions during aqueous workups of a reaction.
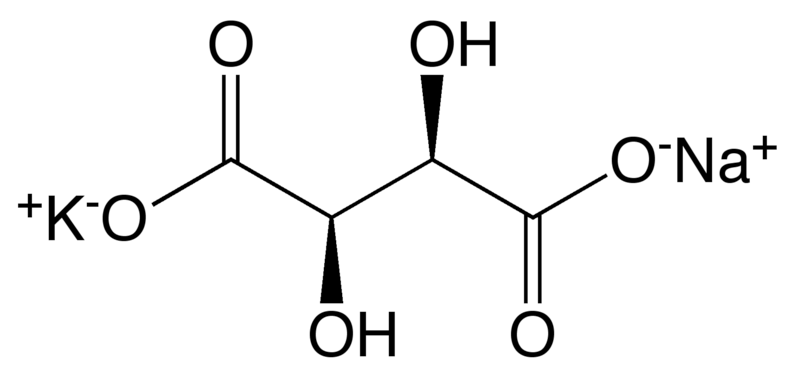
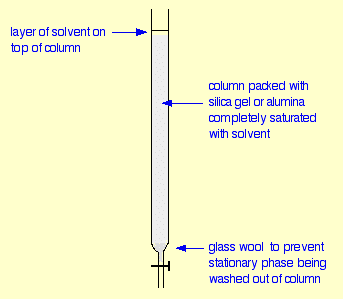
Silica Gel Column Chromatography: This procedure is used to purify the product of both parts of the reaction. The procedure itself involves packing a column with silica gel that will split up the various parts of a mixture, with a high resolution, based on molecular properties.