Brummond, K. M.; Chen, H.; Fisher, K. D.; Kerekes, A. D.; Rickards, B.; Sill, P. C.; Geib, S. J. Org Lett. 2002. 4, 1931-1934.
This article describes in detail the many reaction requirements and formations of unique Pauson-Khand syntheses. The reaction essentially uses an alkene, alkyne, and carbon monoxide to form a cyclopentenone. The researchers discovered an example of an intramolecular Pauson-Khand reaction using a metal catalyst. They contrasted the yield-expectancies of using a rhodium-metal catalyst and a molybdenum-metal catalyst, and found that rhodium is much more effective at producing high yield than other metal catalysts.
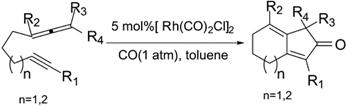
They also analyzed the effect of substituents added to the original molecules on yield, and found out that the Pauson-Khand reaction was generally insensitive to substituent size, implying that many functional groups can be added to the molecules and still undergo the cyclization. Finally, this article gave strong evidence toward the plausibility of forming a 7-membered ring, which is a requirement in the 14-step synthesis of (+)-Ingenol, an anti-cancerous drug derivative.
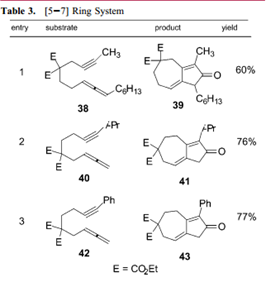
In conclusion, the researchers have delved into the values and limitations of the Pauson-Khand reaction in order to evaluate its use in natural product syntheses. They determined better conditions to produce higher yield, and are still currently researching more efficient ways to perform this highly useful reaction.
Brummond, K. M.; Chen, D.; Davis, M. M. J. Org. Chem. 2008, 73, 5064-5068.
This paper investigates a Rh(I)-catalyzed cyclocarbonylation reaction The overall implications of the paper reveal the general synthetic utility of the Rh(I)-catalyzed cyclocarbonylation reaction for using allenes - ynes to access tricyclic and angular ring systems.
Kitagaki, S.; Inagaki, F.; Mukai, C. J. Synth. Org. Chem. Jpn. 2009, 67, 618-627.
This paper describes the development of several new cycloaddition reactions between an allenyl pi bond and a carbon-carbon multiple bond. One of these reactions is a Pauson-Khand type reaction of allene-alkyne substrates. These substrates, with the aid of of Rh catalyst under a carbon monoxide atmosphere, react to form bicycle[5.3.0] decadienone network.
Shaw, J. T. Nat. Prod. Rep. 2009, 26, 11.
This paper describes various methods for enabling synthesis that are target and diversity-oriented. It includes the Schmidt reaction, a reaction that assembles complex heterocycles. Additionally, it references the Pauson-Khand reaction during a discussion of alkyne/allene reactions and their divergent, transition metal-catalyzed properties.
.jpg)

.jpg)
