What conditions are we to encounter as we sail along the organic seas?
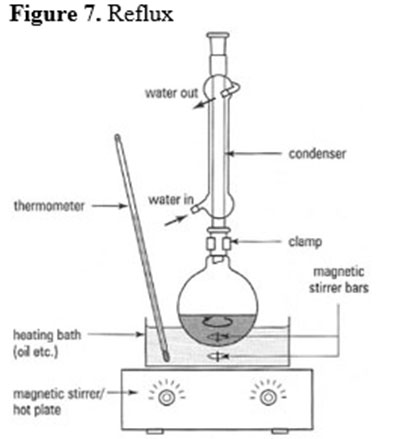
Triethylamine (76 mL, 550 mmol, 10 equiv) and acetic acid (31.4 mL, 550 mmol, 10 equiv) were combined in a sealed tube in a water bath and stirred for 10 minutes. The epoxide 31 (21.3 g, 55 mmol) was added and the tube was sealed. The reaction was heated to 130 °C and proceeded for 16 h. After cooling to room temperature, CH2Cl2 (500 mL) was added to dilute the reaction mixture. The resulting mixture was washed with water (200 mL), 1 M HCl (200 mL), sat. aq. NaHCO3 (200 mL), water (200 mL) and sat. aq. NaCl (200 mL). The organic fraction was dried with MgSO4 and subsequently concentrated. The crude product was purified using flash chromatography (CH2Cl2:acetone 6:1 to 2:1), resulting in the desired regioisomer (product 34) (15.75 g) and the undesired regioisomer (product 35) (7.85 g). In order to obtain a more favorable yield of 34, 35 was recycled by combining 7.85 g of 35 (17.6 mmol), 220 mg of DMAP (1.8 mmol, 0.1 equiv), and 88 ml of toluene (0.2 M). This mixture was heated until refluxing and maintained in this state for 24 h. After cooling to room temperature, the reaction mixture was concentrated in a vacuum. Again, flash column chromatography (CH2Cl2:acetone 6:1 to 2:1) was employed to purify the crude product, resulting in the desired regiosiomer 34 (5.0 g, 84% from 31) and the undesired regioisomer 35 (2.4 g, 10% from 31)1.
Compound 31: IR (neat): 3310, 2936, 1701, 1521, 1173, 1104, 1042, 919, 735 cm-1.
Compound 34: IR (film): 3391, 2973, 2946, 1734, 1654, 1501, 1376, 1238, 1167, 1101, 1032 cm-1.
Compound 35: IR (film): 3380, 2973, 2923, 2845, 1734, 1698, 1664, 1501, 1436, 1375, 1313, 1239, 1053, 1033 cm-1.
Glossary