When you first go to design your experiment, you may feel a little bit nervous

and maybe even tell yourself

So you ask someone around you and they answer

Then you get the idea for your experimental!
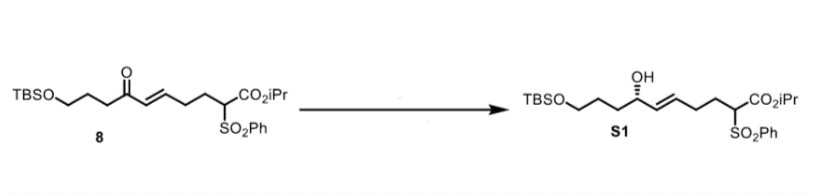
First, you add a solution of (R)-CBS catalyst (3.17g, 10.88 mmol, 0.2 equiv) in CH2Cl2 (100 mL) to a solution of BH3-SMe2 (10 M, 8.2 mL, 1.5 equiv) dropwise over 5 minutes at room temperature. Next, you stir the solution at room temperature for 10 minutes and then cool it to -30°C. You then set it up to make the solution of enone 8 (27g, 54,43 mmol, 1.0 equiv) in CH2Cl2 (50 mL) add dropwise over a period of 24 hours via syringe pump. Look at you go!

After completion of the addition, you stir the mixture for 50 minutes and then quench it with MeOH (40 mL). Next you warm the solution to room temperature and concentrate it in vacuo. Then, you redissolve the residue in MeOH (30 mL) and concentrate it in vacuo once more. Last, you purify the residue via flash chromatography on silica gel (4:1 hexanes:EtOAc) to afford the allylic alcohol S1 (26g, 98%) as a clear oil. Using Mosher ester analysis, you realize that the enantiomeric excess is determined to be >95% and you report H NMR, C NMR, and IR.
Although you've become a bit more sure of your skills, you realize that you still have to get this S1 intermediate to product 10...

At this point you might think running away

is your best option, but you're tougher than that. Thus begins the second experimental.
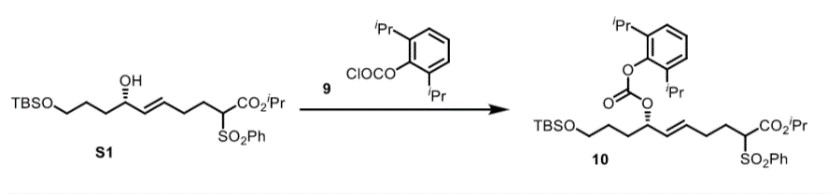
First, you add the allylic alcohol S1 (27.00 g, 54.22 mmol, 1.0 equiv), dissolved in DCM (200mL) and cool it to 0°C. Then, in one portion, you treat each with pyridine (12.7g, 160.7 mmol, 3.0 equiv), diisopropylphenyl chloroformate 9 (19.6 g, 81.33 mmol, 1.5 equiv) and DMAP (0.5 g, 4.09 mmol, 0.05 equiv). You stir the resulting thick white suspension for 3 hours at 0°C and then quench it with 10% aqueous HCl (80mL). The resulting mixture is diluted with water and extracted with Et2O (4 x 100 mL). You wash the combined organics with 2 M NaOH (3 x 100 mL), saturated aqueous CuSO4 (3 x 50 mL), water (100 mL), and brine (100 mL). The remaining solution is dried over MgSO4, filtered, and concentrated in vacuo. You then purify the residue via flash chromatography on silica gel (5:1 hexanes:EtOAc) to afford the desired product allylic carbonate 10 (35g, 95%) as a colorless oil. You report H NMR, C NMR, and IR.
You look around. You've done it. You've successfully purified compound 10. You change your mind about yourself and ask those around you


You feel so confident in your abilities; you've done so much, you're untouchable. You post a new image to your social media, declaring yourself

And you run through the streets yelling, "CATCH ME IF YOU CAN!"




