Use transition state theory to calculate the frequency factor A at 300K for the reaction
H + HBr ® H2 + Br
Additional Information Literature values.† (Note: Most of this information can be obtained from computational chemistry software packages such as Cerius2, Spartan or Cache.)
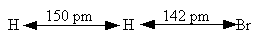
Reactants. H, HBr
H atom (mass) 1 amu
HBr (mass) 80.9 amu
HBr vibration wave number 2650 cm–1
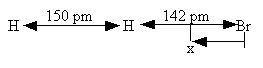
H – Br separation distance = 142 pm
Transition State Complex. H–H–Br
Vibration wave numbers
2340 cm–1
460 cm–1 (degenerate
Separation distances

Solution
The reaction is
H + HBr  H–H–Br ® H2 + Br
H–H–Br ® H2 + Br
The specific reaction rate is
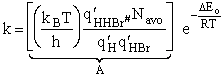
Reactants
Hydrogen
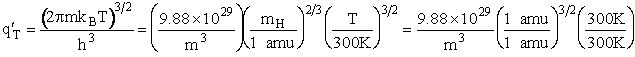
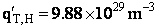
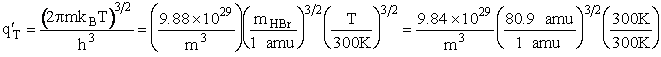
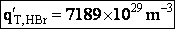
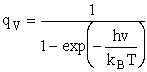
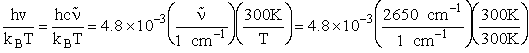
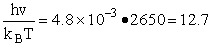
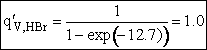
Rotation
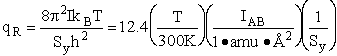
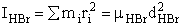
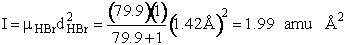
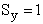
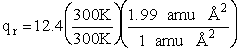
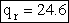
Total partition function
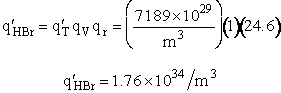
Translation
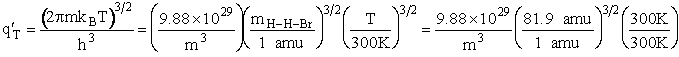
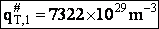
Vibration
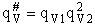
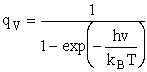
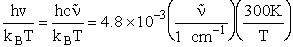
1) u = 2340 cm–1
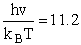
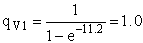
2) u = 460 cm2
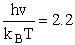
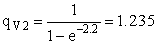
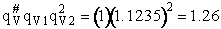
Rotation
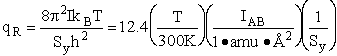
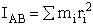
Calculate the rotational partition function,
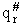 , for the transition
state shown below.
, for the transition
state shown below.
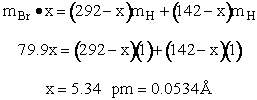
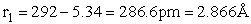
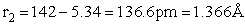
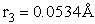
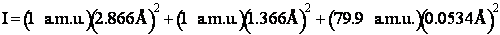
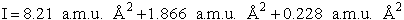
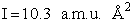
The rotational partition function is
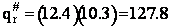
The total partition function for the transition state is

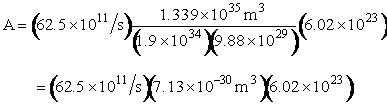
We now calculate the frequency factoring A.

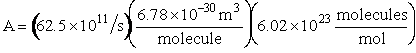
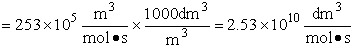
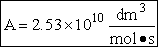
Data From Computational Chemistry
Now let’s calculate A and E using the parameters from cache.
For the reaction:
H + HBr ® H2 + Br
The 3D potential energy surfaces of the reacting particles along the reaction coordinates was calculated using the MOPAC PM3 method:
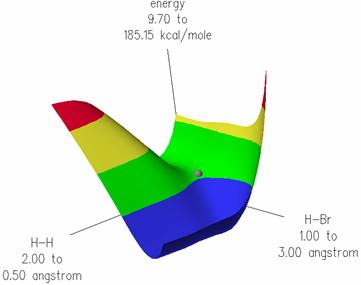
The transition state structure was found at the saddle point, refined by using the DFT/B88-PW91 method as:
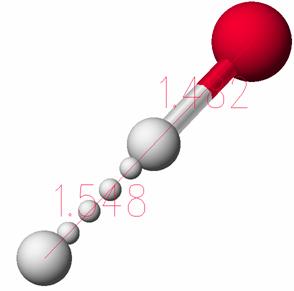
In the transition state, the three atoms are linear and the H-Br distance is 1.48 Ĺ while the H-H distance is 1.55 Ĺ.
The transition state was further proved by vibrational analysis (PM3 FORCE) showing one and only one negative vibration (imaginary frequency of crossing the barrier). Moreover, the negative vibration corresponds to the movement of the atoms on the two reaction coordinates.

Summary of Information from Cache Software
Reactants. H, HBr
H atom (mass) 1 amu
HBr (mass) 80.9 amu
HBr vibration wave number 2122 cm–1
H – Br separation distance = 147 pm
Transition State Complex. H–H–Br
Vibration wave numbers
1736 cm–1
289 cm–1
Separation distances
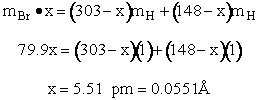
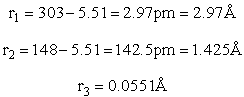
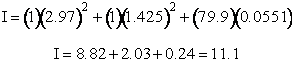
qr = 137.5
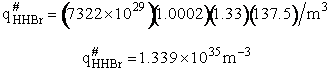
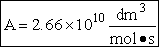
| Chemical |
Heat of formation at 298K (kcal/mol) |
Energy of zero point level (a.u.) |
|
| MOPAC PM3 method |
Experiments |
DFT/B88-PW91 method |
|
| HBr |
5.3 |
-8.71 |
-2574.451933 |
| H |
52.1 |
52.1 |
-0.502437858 |
| H-H-Br Transition state |
59.6 |
N/A |
-2574.953345 |
Therefore, the standard enthalpy of activation is:
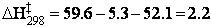 kcal/mol
= 9.2 kJ/mol
kcal/mol
= 9.2 kJ/mol
The intrinsic Arrhenius activation energy is:
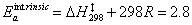 kcal/mol
= 11.7 kJ/mol
kcal/mol
= 11.7 kJ/mol
Barrier height E0 (difference between zero-point levels of activated complexes and reactants) (because the conversion between the a.u. and the kcal/mol units is very large, we need to maintain a high number of decimal point):
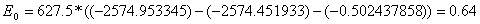 kcal/mol
= 2.7 kJ/mol
kcal/mol
= 2.7 kJ/mol
Return to Transition State Theory