Journal Critique
AIChE J. 16 (1970)
Reaction Overview
Diels Alder condensation 1928 Otto Diels & Kurt Adler Þ Nobel Prize 1950 [Solomon p.520 - Reaction between a conjugated (alternating double and single bonds) and a compound containing a double bond called a dienophile).
• Diels Adler reactions are highly stereo specific
• Diene reacts in s-cis conformation rather than s-trans ("s-" means rotation about a single bond).
Conjugated diene [a 4 p electron system] + Double bond (2-p electron system]® Adduct
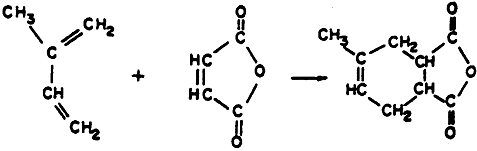
 +
+ 
![]()
A
+
B ![]() C
C
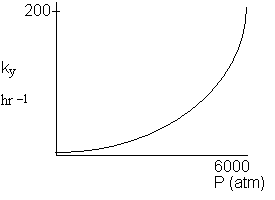
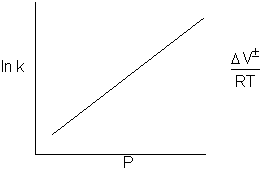
Figure 1: Increase in ky with pressure.
Problems with Previous Work
1) Pressurized after mixing of reactants. Pressurizing caused (adiabatic compression) the temperature to rise during the first part of the reaction.
Solution
Overcame this problem by pre-pressurizing.

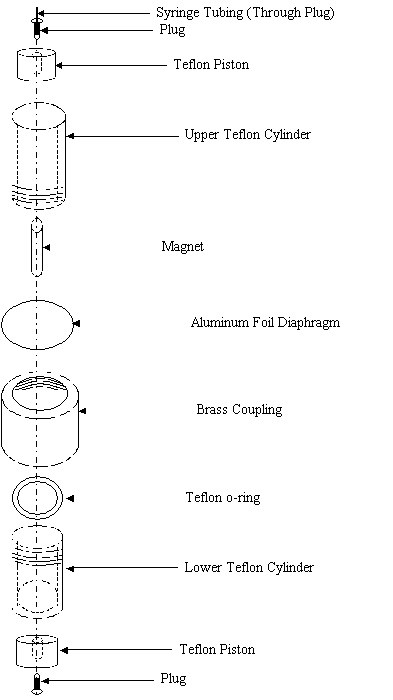
Figure 2: Experimental apparatus. Courtesy of AIChE J. 16 p.766 (1970).
Difficulties
2) Magnet would not break the foil when dropped.
Solution:
Used a current to accelerate it. However, current caused slight electrical heating. Fortunately one could calculate the temperature rise and show it is insignificant.
3) Above 1500 atm Teflon deformed and cell leaked. (Also leaked at high temperature.)
Solution:
Used stainless steel cell.
4) Teflon O-rings leaked in stainless steel cell. Ordinary O-rings swell.
Solution:
Used O-ring of CNR-nitrose rubber, wrapped with Teflon.
5) Isoprene contained dimer impurity which affected the reaction.
Solution:
Reaction mixture distilled in a Podbilniak column
6) Ethyl acetate solvent had small amounts of impurities that interfered with G. C. chromatographic analysis for maleic anhydride.
Solution:
Solvent distilled before use.
Analysis
To
show:
![]()
Solution:
![]()
![]()
Reactants and active intermediates are virtually in equilibrium
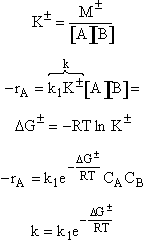
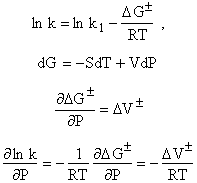
Results
|
P atm 1 170 1000 3000 6000 |
ky hr-1 4.41 5.6 15.7 11.2 1050 |
|
Algorithm
Batch Reactor
Given:
![]()
This equation needs to be derived
Mole Balance
![]()
Rate Law -rA = kCACB
Stoichiometry

![]()
As the pressure on the liquid is increased the liquid is compressed and therefore, CA0, the number moles per unit volume is increased.
Could this explain the increase in ky??
Compression increase P increase CA0. If you look up compression of solvent. Compression about 3%, therefore the increase in liquid concentration by the application of pressure cannot explain the more than two orders of magnitude increases in the rate.
Summaries
Critique Ideas - Don't ask perfunctory questions
1) Did they rinse the reactor between runs?
2) Did they measure the pressure correctly?
Assume researchers are competent.
Socratic questioning is at the heart of critical thinking
When critiquing a journal article, use the six types of Socratic Questions
(1) Questions for clarification: What is ambiguous and needs clarification? What was the basis of calculation?
(2) Questions that probe assumptions: Was the data taken in the reaction rate limited regime?
(3) Questions that probe reasons and evidence: Does the data support the mechanism and rate limiting step?
(4) Questions about viewpoints and perspectives: Are there alternative explanations or interpretations?
(5) Questions that probe implications and consequences: What are the implications and consequences of an assumption or trend being uncertain or invalid?
(6) Questions about the question: Are the results useful? How can these results be applied to other situations?