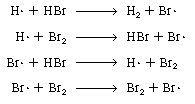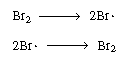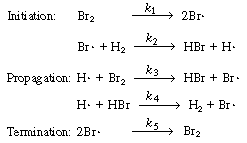|
|
|
|
|
|
|
|
One of the most studied chain reaction is that
between hydrogen and bromine: |
|
|
|
|
|
|
|
|
|
H2 + Br2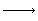 2HBr
2HBr
|
(CD7-1)
|
|
|
|
|
|
|
|
|
In studying this reaction we first deduce the
rate law from reaction rate-concentration data and then formulate a mechanism
and rate equation that are consistent with experimental observations. The
relative rates of the reaction of bromine, hydrogen, and hydrogen bromide
are |
|
|
|
|
|
|
|
|
|
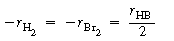
|
(CD7-2) |
|
|
|
|
|
|
|
|
Synthesis of the Rate Law from Experimental Data.
An analysis of the experimental rate data shows the following observations. The
reaction is:
|
|
|
|
|
|
|
|
Experimental
observations
from which to deduce
the rate law
|
|
- First order with respect to the H2 concentration.
- Nearly independent of the HBr concentration at low HBr concentrations, and the
decreases with increasing HBr concentrations at high HBr concentrations.
- Three-halves order in Br2 at low
Br2 concentrations and one-half-order
in Br2 at high Br concentrations.
|
|
|
|
|
|
|
|
|
|
Example CD7-1
Deducing the Rate Law
|
|
|
|
|
|
|
The Mechanism
|
|
|
|
|
|
|
|
We first postulate that the active intermediates are bromine
and hydrogen free radicals Br and
H and
H ,
and . Referring to the denominator of the rate equation and to part 1 in
Text Table 7-1, we see that the active intermediates Br ,
and . Referring to the denominator of the rate equation and to part 1 in
Text Table 7-1, we see that the active intermediates Br and
H and
H collide
with HBr and Br2 o intermediates times two species) delineating
these collisions or interactions are collide
with HBr and Br2 o intermediates times two species) delineating
these collisions or interactions are |
|
|
|
|
|
Propagation steps
|
|
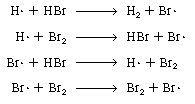
|
|
|
|
|
|
|
|
The last reaction has no effect on the overall reaction because
one Br2and one Br are
produced for every Br2 and Br are
produced for every Br2 and Br that
are consumed. However, it is logical to assume that Br2 and H2
probably react instead of Br that
are consumed. However, it is logical to assume that Br2 and H2
probably react instead of Br and
Br2: and
Br2: |
|
|
|
|
|
|
|
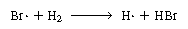
|
|
|
|
|
|
|
|
Note that our proposed scheme of reactions [Equations (CD7-3)
through (CD7-6)] produces one of the active intermediates in each step.
Now we only need a reaction yielding the initial formation of Br and
H and
H .
Experience and, possibly, a good guess tell us that the fractional reaction
order of Br2 in the numerator suggests
that the primary reactive intermediate is Br? formed from Br2
and terminated by the reaction of two bromine free radicals: .
Experience and, possibly, a good guess tell us that the fractional reaction
order of Br2 in the numerator suggests
that the primary reactive intermediate is Br? formed from Br2
and terminated by the reaction of two bromine free radicals: |
|
|
|
|
|
Initiation and termination steps
|
|
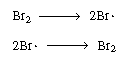
|
|
|
|
|
|
|
|
As a first approximation we shall assume that the mechanism
consists of reactions (CD7-3), (CD7-4), and (CD7-6) through (CD7-8). If
this mechanism does not produce a rate equation that is consistent with
the experimental observations in Equation (CDE7-1.6), we could propose a
different mechanism that might include Equation (CD7-5) together with H2 2H 2H and
similar reactions. The mechanism we first propose is and
similar reactions. The mechanism we first propose is |
|
|
|
|
|
The mechanism
|
|
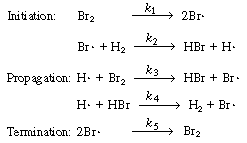
|
|
|
|
|
|
|
|
The specific reaction rates k1
and k5are defined with respect
to Br2. |
|
|
|
|
|
|
|
Example CD7-2
Deriving the Rate Law from the Reaction Mechanism
|
![]() 2HBr
2HBr
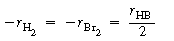
 and
H
and
H