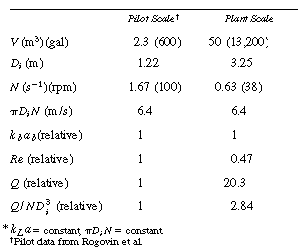
TABLE R7.2-2
1. Choose fermenter volume required based on desired capacity.
Algorithm for fermentor
![]()
2. Choose impeller diameter, Di.
3. Calculate reactor dimension (e.g., DT = tank diameter)
based on geometric similarity, with the
![]()
Alternatively, we could have chosen the tank diameter, DT,
in step 2 and then used Equation (A) to
4. Calculate impeller speed, N.
(![]() DiN)PLANT
= (
DiN)PLANT
= (![]() DiN)AB
DiN)AB
Then
![]()
5. Choose mass transfer correlation for kbab.
6. Calculate gas flow rate using the correlation and setting
(kbab)PLANT = (kbab)LAB
![]()
Then
![]()
7. Calculate power requirement.
7A. An alternate (2) procedure for determining N and Q is to set either
Q/ND = constant or
VS = constant and then determine N from power or
mass transfer correlations. These and other techniques, such as keeping
the power per unit volume a constant, are discussed in Baily and
Ollis.*
* T. J. Bailey and D. Ollis, Biochemical Engineering, 2nd ed., McGraw-Hill, New York, 1987.
TABLE R7.2-3
PHOSPHOMANNAN FERMENTATION SCALEUP*