Example CD7-3
Derive a Rate Law For Competitive Inhibition
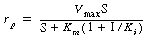
 S)
and (I
S)
and (I E).]
E).]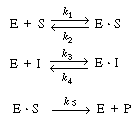
![]()
Example CD7-3
|
|||
| Show that the rate law for the mechanism in Equation (CD7-10) involving competitive inhibition, is | |||
|
|
(CD7-11) | ||
where I is the inhibitor concentration and rp
is the rate of formation of product P. [Hint: Apply the pseudo-steady-state
hypothesis to (E S)
and (I S)
and (I E).] E).] |
|||
| Solution | |||
|
|
|||
| The rate of formation of product P corresponding to the last step in this reaction sequence is | |||
|
|
(CDE7-3.1) | ||
| The uncomplexed or free enzyme concentration is | |||
|
|
(CDE7-3.2) | ||
| Using the pseudo-steady-state approximation for the enzyme-substrate complex yields | |||
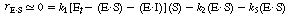 |
(CDE7-3.3) |
| For the enzyme-inhibitor complex, |
|
|
(CDE7-3.4) |
| Dividing Equation by k1(S) and Equation (CDE7-3.4) by k3(I) and rearranging each, we obtain | |||
|
|
(CDE7-3.5) (CDE7-3.6) |
||
Subtracting Equation (CDE7-3.5) from (CDE7-3.6) and solving
for E I,
we find that I,
we find that |
|||
|
|
(CDE7-3.7) | ||
After substituting Equation (CDE7-3.7) into (CDE7-3.5) to
solve for E S, S, |
|||
|
|
(CDE7-3.8) | ||
we substitute for E S
in Equation (CDE7-3.1) to obtain the rate law for the inhibition of the
competitive type: S
in Equation (CDE7-3.1) to obtain the rate law for the inhibition of the
competitive type: |
|||
|
|
(CD7-11) | ||
| WHERE | |||
|
|
|||
Letting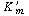 . . |
|||