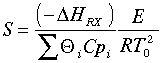
In Barkelew's orginial article he gives an example calculation. The approximation is in calculating S:
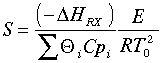
If only A and inerts enter and the mole fraction, yA0, of A is 0.1, then QI = 0.9/0.1=9.
![]()
![]()
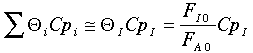
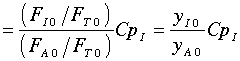
Further, the author assumes
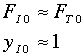
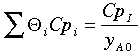
Another way to get the same result is if
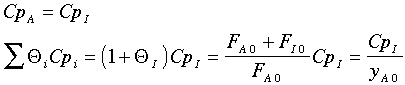
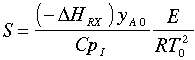
There is also an error in the article in calculating (-rA0). The author forgot to multiply k by yA0. (i.e., -rA0=kyA0).
Back to Runaway Reactions in PFRs