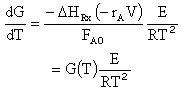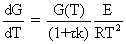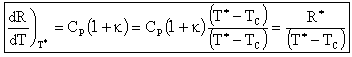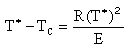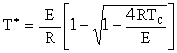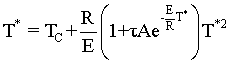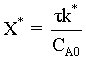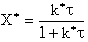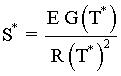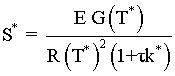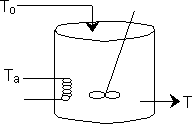
We are going to consider a CSTR which has a runaway reaction when it goes to the upper steady state which is at an unacceptably high temperature, such as the propylene glycol reaction discussed in Example 8-5 in the text. This transition to the upper steady state will occur when the point of tangency between R(T) and G(T) disappears and only the upper steady state exists.
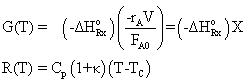 |
(8-60) (8-59) |
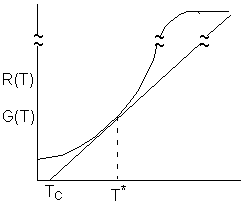
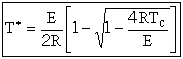
At the point of tangency T = T*,
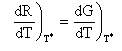
For a zero order reaction
|
(1) |
For a first order reaction
|
For a zero order reaction | (2)
|

This equation is Eqn. (8-77) on p.498 of the text. Solving for T*
| (4) |
If ![]() the point of tangency will disappear and the system will be considered unstable
because it moves to the upper steady state. We refer to this movement to the
upper steady state as runaway. Combining the Eqn. directly below (8-74)
and Eqn. (8-75) in the text.
the point of tangency will disappear and the system will be considered unstable
because it moves to the upper steady state. We refer to this movement to the
upper steady state as runaway. Combining the Eqn. directly below (8-74)
and Eqn. (8-75) in the text.
Suppose the heat exchange term UA is decreased. As a result k will decrease and we will no longer
have a point of tangency and as a result the CSTR will runaway to the upper steady state.
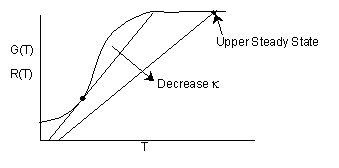
As a result, this decrease in k will cause
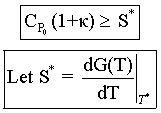
|
Procedure:
|
If, for example,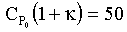 then
to the right of TC1,
then
to the right of TC1, 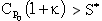 and the reactor is stable, so we can increase either To or Ta in this region up to TC1
and the reactor is stable, so we can increase either To or Ta in this region up to TC1
if we decrease 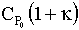 to say
to say 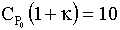 , then we see we have a narrower operating range over which To and Ta may by increased.
, then we see we have a narrower operating range over which To and Ta may by increased.
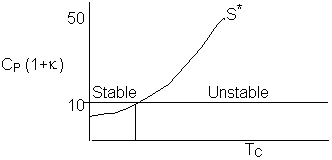
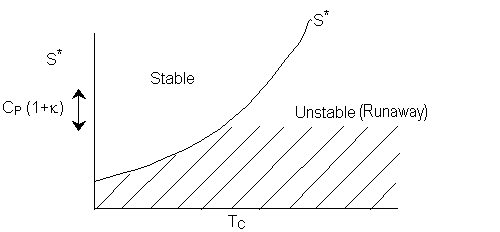
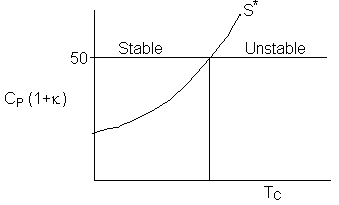
Consequently the line S* divides the place into regions for values
of ![]() that will be either stable or unstable for a given Tc. Similarly, for a fixed TC1 the region of stability is
that will be either stable or unstable for a given Tc. Similarly, for a fixed TC1 the region of stability is
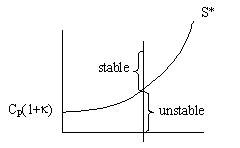
Recall 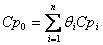 , so we can change the region by changing the amount of inerts.
, so we can change the region by changing the amount of inerts.