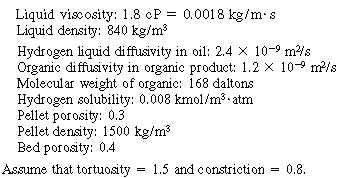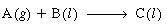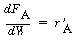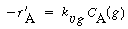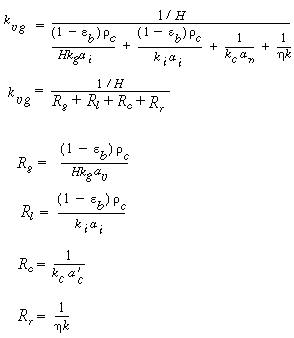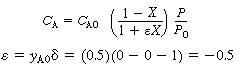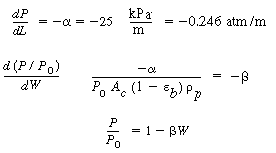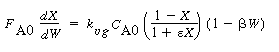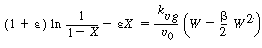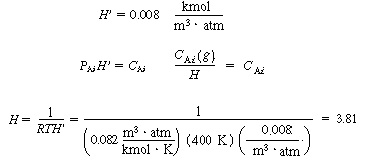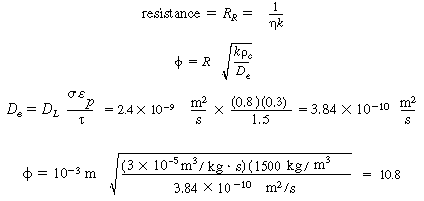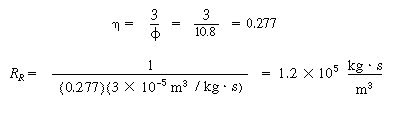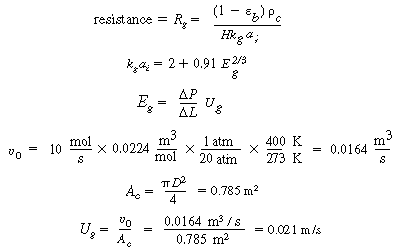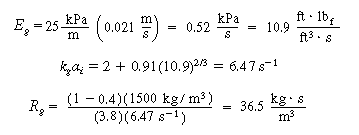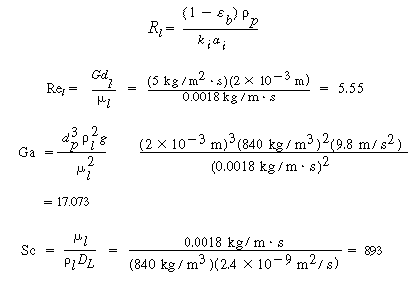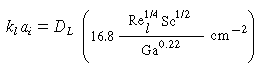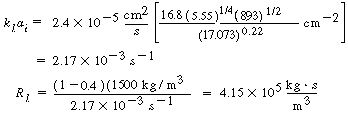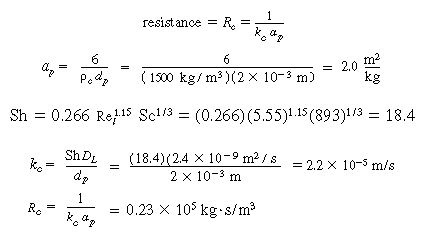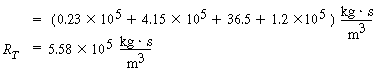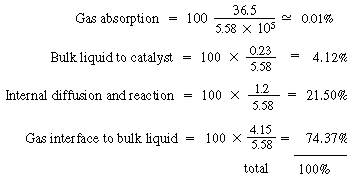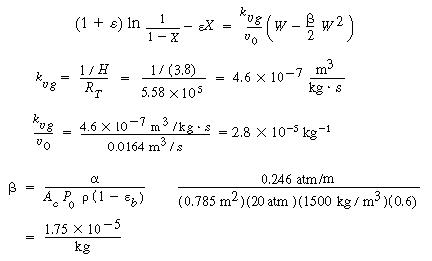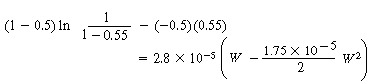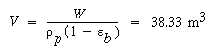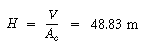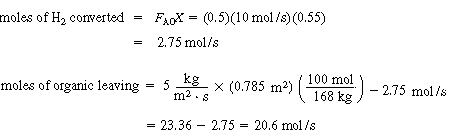|
|
The hydrogenation of an unsaturated organic is to be carried out in
a trickle bed reactor packed with 0.20-cm-diameter spherical catalyst particles.
|
|
|
|
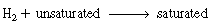
|
|
|
|
The reaction in the pellet is first-order in both hydrogen and the
organic. Hydrogen and nitrogen are fed in equimolar portions at a total pressure
of 20 atm and a total molar rate of 10 mol/s. The reactor diameter is to be 1.0
m. The superficial liquid mass velocity is 5.0 kg/m 2 s. The corresponding pressure
gradient through the bed is 25 kPa/m. s. The corresponding pressure
gradient through the bed is 25 kPa/m.
As a first approximation, assume that the concentration of organic is constant and
the pseudo-first-order specific reaction rate is 3 x 10 -5
m 3 / kg cat. s at 400 K. s at 400 K.
|
|
|
|
(a) For each transport step, determine its fraction of the
total resistance to mass transport and reaction.
(b) Calculate the catalyst weight necessary to achieve 55% conversion of the
hydrogen.
|
|
|
|
Additional information:
|
|
|
|
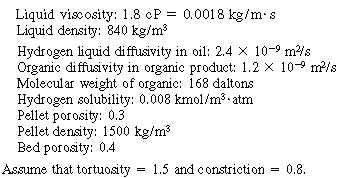
|
|
|
|
(a) Let A = H 2
, B = unsaturated organic, and C = saturated organic:
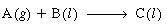
|
|
|
|
1.Mole balance on H
2
(A):
|
|
|
|
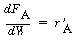
|
(CDE12-1.1)
|
|
|
2. Rate law.Assuming a constant liquid reactant concentration
for low conversion of B,
|
|
|
|
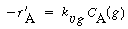
|
(CDE12-1.2)
|
|
|
with
|
|
|
|
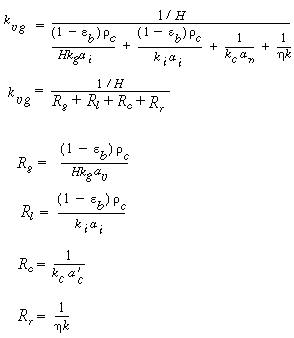
|
(CDE12-1.3)
|
|
|
3 .Stoichiometry. The isothermal gas-phase
concentration is
|
|
|
|
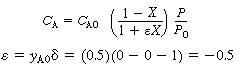
|
(CDE12-1.4)
|
|
|
4. Pressure drop:
|
|
|
|
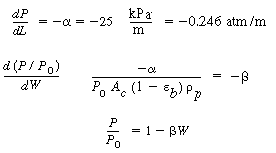
|
(CDE12-1.5)
|
|
|
5. Combining yields
|
|
|
|
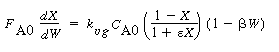
|
(CDE12-1.6)
|
|
|
Integrating gives us
|
|
|
|
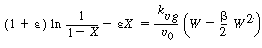
|
(CDE12-1.7)
|
|
|
6. Evaluating the parameters:
A.Solubility
|
|
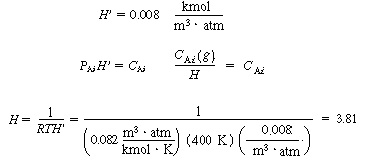
|
(CDE12-1.8)
|
|
|
B. Internal diffusion and reaction
|
|
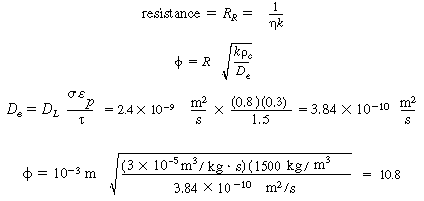
|
(CDE12-1.9)
(CDE12-1.10)
|
|
|
For large values of the Thiele modulus,
|
|
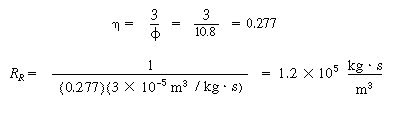
| (12-1)
(CDE12-1.11)
|
|
|
C. Gas absorption
|
|
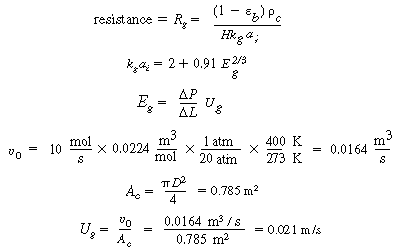
|
(CDE12-1.12)
(TCD12-1A)
|
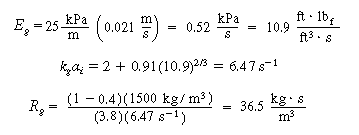
|
|
|
|
D. Transport from gas-liquid interface to bulk liquid
|
|
|
|
|
|
|
|
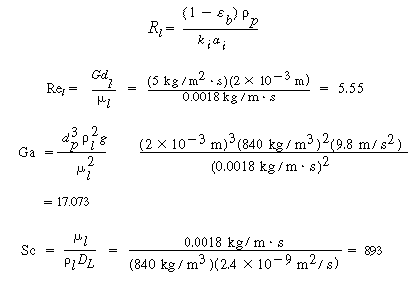
|
(CDE12-1.13)
|
|
|
From the correlation for organic liquids,
|
|
|
|
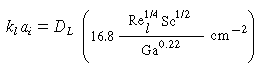
|
(CDE12-1.14)
|
|
|
It has been noted 5
that this correlation gives a mass transfer coefficient that is too low
|
|
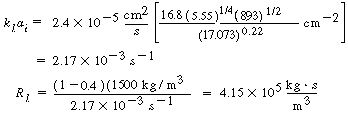
|
|
|
|
E. Resistance from bulk liquid to catalyst
|
|
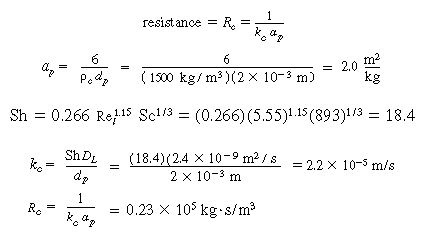
|
(CDE12-1.15)
(TCD12-IF)
|
|
|
F. Total and percentage resistances
|
|
|
|
R T =
R c + R l
+ R g + R R
=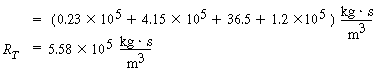
|
(CDE12-1.16)
|
|
|
Individual resistances:
|
|
|
|
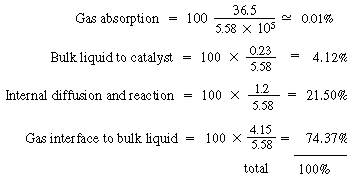
|
|
|
|
(b) Calculate catalyst weight
|
|
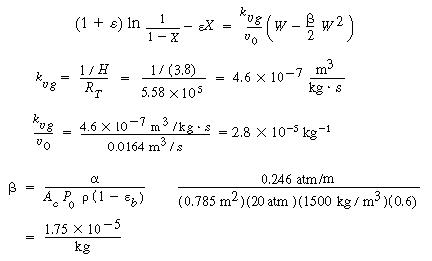
|
(CDE12-1.7)
(CDE12-1.17)
|
|
|
Substitution yields
|
|
|
|
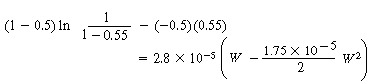
|
|
|
|
Solving, for W, we obtain
|
|
|
|
W = 34,500 kg
|
|
|
|
The reactor volume corresponding to this catalyst weight is
|
|
|
|
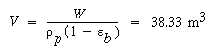
|
|
|
|
The total height of the reactor
|
|
|
|
|
|
|
|
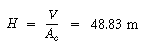
|
|
|
|
Four 1-m-diameter towers each 12.2 m in height connected in series
will be sufficient.
|
|
|
|
Checking assumption of constant C B
|
|
|
|
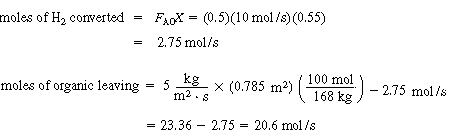
|
|
|
|
Consequently, our assumption that the concentration of organic is
essentially constant was valid.
|
|
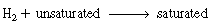
 s. The corresponding pressure
gradient through the bed is 25 kPa/m.
s. The corresponding pressure
gradient through the bed is 25 kPa/m. s at 400 K.
s at 400 K.