 4-Pentenal
4-Pentenal 4-Pentenal
4-Pentenal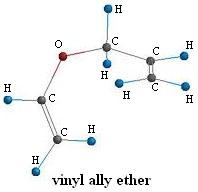
Computational chemistry has not yet advanced to the point where large-scale plants can be designed solely on a theoretical basis. However, the calculated quantities can help you:
- Decide if a reaction system is even feasible for further development, before you spend a lot of money and time doing experiments
- Determine whether experimental results (which aren't always correct and reproducible!) are consistent with what you expect for the system at hand
- Help identify an improved process (e.g., a new catalyst)
You will now begin thinking on a molecular level
If we take a close look at the reaction above we can see that three things happen:
- A carbon-oxygen bond is broken
- A carbon-carbon bond is formed
- A carbon-oxygen double bond is formed
Since we start and end with the same atoms in the molecule the reaction must be intramolecular. So if we just bring the two free ends of the molecule together and "push" some electrons around like this:
Figure 3.
The arrows that you see in figure 3 represent the movement of the electrons as the molecule transitions from the reactant to the transition state.
And thus the transition state would be:
Figure 4.
In figure 4 the dashed lines represent the bonds being formed and broken in the transition state.