Ozone Depletion
Web Module
Anjan K. Chakrabarti
Alejandra De Obeso
Dr. Nihat M. Gürmen
Prof. H. Scott Fogler
 Reactions
Reactions
![]()
![]()
![]()
Chapman Cycle
Without the interference of halogens and other species that catalyze the destruction of ozone, the ozone layer maintains a steady state concentration via the Chapman Cycle.
Both O2 and O3 absorb light in this reaction scheme, forming atomic O, which destroys and creates ozone. This is a natural cycle that does not affect the overall concentration of ozone.
Photolysis of Chlorofluorocarbons (CFCs)
CFCs, the substances discussed in the background section, release chlorine by absorbing light. To demonstrate this reaction, we will be looking at CCl2F2 (also called CFC-12), where the C-F bond (dissociation energy 110 kcal mol-1) is much stronger than the C-Cl bond (dissociation energy 76 kcal mol-1), which allows the C-Cl bond to break at longer wavelengths.
The significance of this reaction is that it produces chlorine atoms. CFCs alone are harmless to the ozone layer: it is the chlorine atoms released by photolysis that are harmful.
Catalytic Destruction of Ozone by Chlorine
Chlorine released from CFCs participates in a catalytic chain reaction which results in the destruction of O3. There are two specific mechanisms of interest, which lead to the net destruction of ozone.
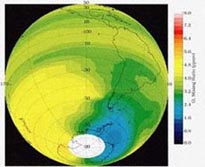
Using the mentioned reactions in a concise system, we can develop a model for the catalytic destruction of O3 in the ozone layer by chlorine released from CFCs. The following image sums up the reactions discussed in this section:
See QuickTime Movie of Ozone Destruction due to chlorine released from CFCs
(Embedded, Direct)
.jpg)