4th Edition, 3rd Printing Typos
Note: Typos are currently being added for the 3rd printing
Throughout the Text
Throughout the text, the following units: hr (time), lb (mass), C (temperature), F (temperature), R (temperature)
should read: h (time), lbm (mass), °C, °F, °R
| Reads | Should Read |
| hr (time) | h (time) |
| lb (mass) | lbm (mass) |
| C (temperature) | °C (temperature) |
| F (temperature) | °F (temperature) |
| R (temperature) | °R (temperature) |
Expression such as mol/m3•s and mol/m3/s are used interchangeably throughout the text
Chapter 1
Page 21, the equation in the margin under the graph should read
CA = CA0exp(-kV/υ0)
Page 29, P1-1A part (b), should refer to Figure 1-2, not 1-1
Page 29, Place Web Hint Icons for P1-1A part (d) and P1-3A part (b)
Page 30, P1-8A part (e), the last r′A should read r″A in the question
Page 31, P1-11B, near bottom pencillin should read penicillin
Page 31, P1-12A, lb/yr should read lbm/yr
Chapter 2
Page 71, Top equation, denominator should read ... -rA(X = 0) ... for FA0/[-rA(X = 0)], insert "A" for the first rate.
Page 73, P2-2A Part (b), also look at the back of the book on pages 1046 and 1047 about learning styles
Page 73 P2-4A Part (b), divided equally to each reactor should read between the reactors
Page 74, P2-7B Part (c), 10.5 dm3 should read 105-dm3
Page 75, P2-8B Part (e), -rs should read -rS and Cc0 should read CC0
Chapter 3
Page 85, Table 3-1 Part B Reaction (1), NH4CL should be NH4Cl
Page 91, Equation (C-9) ΔHRx should read ΔH°Rx
Page 97, under part b, 8.314 kJ/mol•K should read 8.314 J/mol•K
Page 113, on the last line using Equation (3-42) should read using Equation (3-45)
Page 118, Equation (E3-5) should read (E3-5.2)
Page 124, Equation (C-9) ΔHRx should read ΔH°Rx
Page 125, Bottom line of chart should include and θi=Fi0/FA0.
Page 129, under the Vibrations section, qV should read q′V
Page 129, under the Rotation section, qR should read q′R
Page 130, on the horizontal axis label (E=1/2uU2R) should read(E=1/2mU2R)
Page 131, P3-2A part (a) - 4th line, add the word energy to activation,
should read "Next write a paragraph describing the activation energy, ..."
Page 131, P3-2A part (c) - 2nd line, change spelling to glyceryl stearate
Page 131, P3-2A part (e) - 2nd sentence, should read "Plot Equation (E3-5.2) as (1/-rA) versus X up to X = 0.99. What did you find?"
Page 136, P3-13B introduction sentence,
change the reactants and products to ortho-nitroaniline and ortho-nitrochlorobenzene respectively
should read "The formation of ortho-nitroaniline (an important intermediate in dyes—called fast orange) is formed from the reaction of ortho-nitrochlorobenzene (ONCB)
and aqueous ammonia. (See Table 3-1 and Example 9-1.)"
Page 137, P3-15B, part (b) - CAO should read CA0
Page 137, P3-16B, part (b) - Equation for KC should read KC=0.25(mol/dm3)2.
Page 140, CDP3-EB, the reaction should read
C6H5COCl + 2NH3 → C6H5CONH2 + NH4Cl
Page 140, CDP3-JB, the reaction should read
3SiH4(g) + 4NH3(g) → Si3N4(s) + 12H2(g)
Page 140, CDP3-KB, the reaction should read
CH4(g) + 2Cl2(g) → CH2Cl2(g,l) + 2HCl(g)
Chapter 4
Page 144, line 6 in grey box, should read "batch data", not "date"
Page 147, Figure 4-2 Equation (B) at the bottom, should read
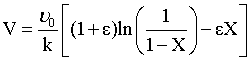
Page 176, Line 7 delete isomerization, should read the second order reaction
Page 185, First equation, 0.1414ft2 should read 0.014142
Page 185, Boxed equation, 0.00165lbm-1 should read 0.0165lbm-1
Page 195, Top Line, Section 4.6 should read Section 4.5
Page 195, Horizontal axes on Figures E4-6.1 and E4-6.2 should read W(lbm)
Page 225, The last reversible reaction is missing the liquid states, should read
Page 234, P4-2B, part (b) - the same composition of reactants should read the same concentrations of reactants
Page 235, P4-2B, part (m), in the third line- each of the regimes, nucleation, growth should read each of the regimes: nucleation, growth
Page 237, part (e) - Table 4-1 should read Table 4-3
Page 237, P4-6B, part (b) - FAO should read FA0
Page 240, In the first line and following equation, ΔHRx should read ΔH°Rx
Page 240, Fourth line, Equation (C-8) should read Equation (C-9)
Page 240, P4-10C, part (a) - delete the word concetration
Page 242, P4-16A, m Xylene should read meta-Xylene.
Page 242, P4-16A, part (b) - τPFR should read τCSTR.
Page 242, P4-18B, Second paragraph, line 4, Equation(4-33) should read Equation (4-29).
Page 243, P4-19, should read P4-19C.
Page 245, under additional information, the Bulk density should have units of kg/dm3.
Page 246, P4-23B, part (b) - 2 mol/min should read 200 mol/min.
Page 246, P4-23B, part (b) - use Table 4-3.
Page 246, P4-24C, line 6, above 35 ° C should read above 37 ° C.
Page 246, P4-24C, after the last sentance add (tc + te + tf)=3h.
Page 247, P4-26B, in the third to last line, kCH2 should read kH2.
Page 248, P4-32B, in the chemical equation, C4H9 should read C4H9OH.
Page 248, P4-33C, 3rd Ed. P4-29 should read 3rd Ed. P4-29B.
Chapter 5
Page 266, the horizontal label for Figure E5-1.3, –dCA should read CA.
Page 270, the horizontal label for Figure E5-2.1 should read t(min).
Page 279, the horizontal label for Figure E5-4.2 should read CHCl,0(mol/dm3).
Page 295, P5-4B,the last line before the chart, mol/dm3 should read mol/cm3.
Page 295, P5-4B, part (a) - constant k should read constant k1.
Page 296, part (a) should read Determine the reaction order and specific reaction rate constant.
Page 296, P5-6B, part (a) - reaction rate should read reaction rate k.
Page 297, in the first chart, 0.1 should read 1.0.
Page 297, the sentance after the first chart, should read The following concetration-time data were obtained for an initial methanol concentration 0.01 mol/dm3 and an initial triphenyl concentration of 1.0 mol/dm3.
Page 297, in the second chart, 0.01 should read 0.1.
Page 300, in the chart, C2H4Br should read C2H4Br2.
Page 302, CDP5-FB, in the chemical equation, ASH3 should read AsH3.
Page 302, CDP5-FB, 3rd Ed. P5-6 should read 3rd Ed. P5-6B.
Chapter 6
Page 313, all CAp should read CA*.
Page 313, in the first box, 0.112 dm3/mol should read 0.112 mol/dm3.
Page 325, in form (1) Xa should read Xa and XIIIa should read XIIIa.
Page 334, line 3, i.e., r3NO2 should read i.e., r3O2.
Page 334, the middle part of (E6-5.22) should read = O2+ r2O2+ r3O2.
Page 343, line 2, 15.23 mol/h should read 15.23 lb mol/h.
Page 361, P6-1A, See Problem P4-1 should read See Problem P4-1A.
Page 362, Example 6-7, first, Load the Living Example Problem from the CD-ROM.
Page 362, part (l) - See Problem P6-22 should read See Problem P6-22C.
Page 369, P6-16B, part (b) should read A CSTR.
Page 370, P6-19C, part (d) should read Repeat (b) and (c) for the methanol reaction given in P6-9C (h) with Pi in bar and -r1CH2OH and -r2CH2O are in bar/s.
Page 371, P6-21B, Additional Information, line 5 should read k3E= 5.0 dm3 / mol2 • kg cat • min.
Page 373, CDP6-24B, the second chemical equation should read C4H2O3 + 3O2 → 4CO2 + H2O.
Chapter 7
Page 404, line after equation (7-29), (see Problem P7-10) should read (see P7-10B(a)).
Page 426, equation 7-64, delete the minus sign on the right side of the equation.
Page 427, equation 7-69 A should read 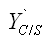 = (mass of substrate to form new cells)/(mass of new cells formed).
= (mass of substrate to form new cells)/(mass of new cells formed).
Page 453, Figure B, the vertical axis label should read CCA (mg/dm3).
Page 454, P7-1A part (c) - in the chart 0.066 should read 0.065.
Page 460, P7-12B, the reaction should not be reversible, it should read E + S → P + E.
Page 463, P7-17B, Problem P7-16 should read Problem P7-16B and μmax= 1 should read μmax= 1.0 h-1.
Page 463, P7-18B, KM= 4 should read KM=4 g / dm3.
Page 463, P7-19B, line 8, CSO=50 g / dm3 should read Cs0= 50 g / dm3.
Page 464, P7-20C, the second equation under "the growth laws are" should read rgX2= μ2CX2.
Page 465, P7-22C, part (e) - Cs0=2.0/dm3 should read Cs0= 2.0 g / dm3.
Page 465, P7-23A, last line, 2 dm3/s should read 2 dm3/h.
Chapter 8
Page 476, in equations T8-1.A and T8-1.B, ΔHRx should read ΔH°Rx.
Page 478, in equation T8-1.B, ΔHRx should read ΔH°Rx.
Page 479, Top Equation needs to be divided by FA0, should read
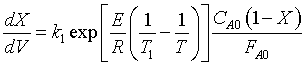
Page 506, in equation E8-5.8 ΔH°Rx should read ΔHRx.
Page 507, in the box, CPA = 163 J / mol / A • K should read CPA = 163 J / mol A / K.
Page 508, in Figure E8-5.2, T = 1035 K should read T0 = 1035 K.
Page 514, Table E8-6.1, the heading for the first column should read T(K).
Page 519, in the equation in the margin, ΔHRx should read ΔH°Rx.
Page 551, Figure E8-11.1, the vertical axis label has units of J/mol.
Page 551, Table E8-11.2, the column headings should read T(K), CA(mol/dm3), CB(mol/dm3), and CC(mol/dm3).
Page 557, B. 1) part (b) - the right side of the equation should read U(T(R,z)-Ta).
Page 560, delete the R in TR in equations 8-100 and E8-12.9
Page 564, in the top graph, -ΔHRxX should read -ΔH°RxX.
Page 565, under ODE SOLVER ALGORITHM, in the second equation on the left, Ua/ρc should read Ua/ρb and in the third equation on the right, UA/ρb should read Ua/ρb.
Page 568, P8-1A, See Problem P4-1 should read See Problem P4-1A.
Page 569, P8-2A, part (i) - ΔHRx should read ΔH°Rx.
Page 570, part (m) - in the third equation on the rigth, Ua/ρB should read Ua/ρb
Page 571, part (i) - at 303 and should read at 303 K and
Page 572, P8-4C, part (a) - See Problem P9-3 should read See Problem P9-3B.
Page 573, Table P8-5, the Molecular weight of Component C should read 111.
Page 574, in the first line replace all 273 with 273 K.
Page 574, P8-7B, Use the data and reaction in Problem 8-6A.
Page 574, P8-7B, part (c) - in the last line, 1 should read 10.
Page 574, P8-7B, part (f) - ΔHRx should read ΔH°Rx.
Page 574, P8-7B, part (f) - add the sentance Choose Ta0 = 450 K.
Page 575, P8-9B, Use the data in Problem 8-8B.
Page 575, P8-9B, part (e) - See Preface B.2 and B.3 should read See Preface, B.2, B.3 and http://www.engin.umich.edu/scps.
Page 576, part (c) and additional information - ΔHRx should read ΔH°Rx.
Page 576, P8-11C, part (b) - P8-9(a) should read P8-9B(a).
Page 578, Additional Information, add CC = CS0YC/SX, change to CPS = 5 J / g / K , change to CPc = 5 J / g / K , and change ΔHRx to read ΔH°Rx.
Page 579, Additional information - ΔHRx should read ΔH°Rx.
Page 580, Additional information - ΔHRx should read ΔH°Rx.
Page 582, P8-21C, line 2, P8-2(n) should read P8-2A(n).
Page 583, part (a) should read Problem P8-6A.
Page 583, part (b) should read Problem P8-9B.
Page 583, before Additional information, delete E = ?? cal/mol.
Page 582, Addtional information, ΔHRx1A should read ΔH°Rx1A and ΔHRx2B should read ΔH°Rx2B.
Page 585, Addtional information, ΔHRx1O should read ΔH°Rx1O and ΔHRx3O should read ΔH°Rx3O.
Page 585, P8-26C, last sentance, the subscript S/BT should read St/BT.
Page 586, Addtional information, ΔHRx1EB should read ΔH°Rx1EB, ΔHRx2EB should read ΔH°Rx2EB and ΔHRx3EB should read ΔH°Rx3EB.
Page 586, the last line should read The temperatre is in Kelvin and Pi is in atm.
Page 587, P8-31B, 3rd Ed. P8-28 should read 3rd Ed. P8-28B.
Chapter 9
Page 623, E8-5.6, ΔHRx should read ΔH°Rx.
Page 633, P9-1A, Problem 4-1 should read Problem 4-1A.
Page 635, line 4, Problem 8-3 should read P8-4C.
Page 635, P9-5B, Problem P8-5 should read Problem P8-6B.
Page 637, P9-8B, Additional information, CPS = 74 J/g/K should read CPS = 5 J/g/K.
Page 638, line 1, add units of kg/m3.
Page 638, line 2, ΔHRx should read ΔH°Rx.
Page 638, line 3, CPC = 74 J/g/K should read CPC = 4.2 J/g/K.
Page 641, P9-13B, should read Apply the different types of controllers to the reaction in Problem P9-11B.
Page 641, P9-15B, Rework Problem P9-3 should read Rework Problem P9-3B.
Page 641, P9-15B, UA should be equal to 10,000 Btu/h•ft2•K.
Page 642, part (e) - all temperatures are in °R and CA has units of lb mol/ft3.
Page 642, Additional information, ΔHRx should read ΔH°Rx.
Page 642, P9-17B, Problem 8-6 should read Problem 8-6A.
Page 642, P9-18B, Problem P8-34B should read Problem P8-33B.
Page 643, in the boxed equation the exponent should read -E/RT.
Chapter 10
Page 720, in Figure E10-6.1 the units for CA are (mol/dm3).
Page 723, in Figure 10-31 the third column heading should read A(h-1).
Page 731, the first sentence should read Differentiating, we can find a relation between the time the catalyst particle has been in the STTR and reached a height z which we can use to find the activity a.
Page 734, under part 6, the equation SiH2 + S → SiH2•S should read SiH2(g) + S → SiH2•S.
Page 739, in the first line the units of r`A, should read mol/kg cat•s and the the units for PH should read atm.
Page 734, part (g) - k = 120 should read k = 120 h-1 and kd= 12 should read 12 dm3/mol•s.
Page 744, P10-10C, part (c) - i-octane should read i-octene.
Page 753, under additional information, ΔHRx should read ΔH°Rx.
Chapter 11
Page 758, in the figure at the bottom of the page, the top right point should read x+Δx,y+Δy,z+Δz, and all uppercase X,Y, and Z's in the figure should be lowercase.
Page 782, in the middle of the page, kc = mass transfer coefficient = DAB/δ (s-1) should read kc = mass transfer coefficient = DAB/δ (m/s).
Page 785, the y axis label on Figure 11-10 should read φ JD.
Page 803, part (e) - kinetic should read kinematic and in the equation, there should be a K after -4000.
Page 803, part (f) - the first sentence should read Derive the three equations on page 772.
Page 805, P11-6B, part (a) - DAB = 0.18 x 10-5 m/s should read DAB = 1.8 x 10-5 m/s
Page 806, part (a) - the equation should read
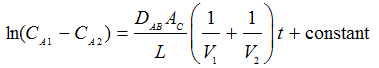
Page 806, part (b) - the equation should read
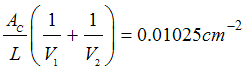
Page 807, P11-11B, under additional information, ΔHRx should read ΔH°Rx.
Chapter 12
Page 842, in the middle of the page, Rg = gas constant, kJ/mol•K should read Rg = gas constant, 8.314 J/mol•K
Page 847, in the box on the left of the page, kc`` should read kc.
Page 857, P12-4B, part (d) - T = 400 should read T = 400 K.
Page 861, in equations P12-13.1 and P12-13.2 and under additional information, ΔHRx should read ΔH°Rx.
Page 861, P12-16B, part (e) - 527°C should read 227°C.
Chapter 13
Page 883, in E13-2.2 the second integral should read tE(t)dt
Page 900, in the middle of the page, τ = 1 should read τp= 1.
Page 911, the x axis label in Figure E13-5.1 should read t(min).
Page 916, in Figure 13-25, on the bottom right, V = V0 should read V = V.
Page 927, in E8-4.9 -ΔHRx should read -ΔH°Rx.
Page 929, the x axis labels for Figures E13-9.1 and E13-9.2 should read t (min) and the y labels should read E(t) (min-1)
Page 936, P13-2A, part (f) ΔHRx should read ΔH°Rx.
Page 939, P13-6B, the y axis labels of Figure P13-6B (a) and (b) should read E(t) (min-1).
Page 939, P13-8B, part (b) - 300 < T < 350 K should read 300 K < T < 350 K.
Page 940, under additional information, E/R = 20,000 should read E/R = 20,000 K.
Page 941, P13-14C, under additional information, ΔHRx should read ΔH°Rx.
Chapter 14
Page 960, in Figure 14-8 (a) -DAB should be divided by U.
Page 964, in the last paragraph (DaU/dt) should read (Da/Udt)
Page 972, the first column in Table E14-2.2 should read
t (min)
C(t) (mol/dm3)
E(t) (min-1)
tE(t)
t2E(t) (min)
Page 989, the x axis label for Figure E14-5.1 should read t (min).
Page 995, R14.2, Real Reactor Modeled in an Ideal CSTR with Exchange Volume should read Real Reactor Modeled two Ideal CSTRs with Exchange Volume.
Page 999, P14-12C, part (b) - P12.2 should read
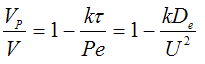
Page 1001, P14-15B, For 10 ≤ t should read For 10 min ≤ t
Page 1003, the x axis labels for Figures P14-22 and P14-23 should read t (min).
Page 1004, P14-24B the y axis label in the figure should read E(t) (min-1).
Page 1004, the third problem on the page should be P14-26B and the y axis label for figure should read C (mM).
Page 1006, the last line of the quote should read But it is perhaps, the end of the beginning.
Appendices
Page 1017, in the last line, 0.062 lb•mol/ft3 should read 0.062 lb mol/ft3.
Page 1018, under Pressure, 1 psi should read 1 psia.
Page 1018, the second line under Temperature, R should read °R.
Page 1018, under work, part B. - 1 Newton/m2 • m3 should read (1 Newton/m2) • m3
Page 1022, all the p and c subscripts for the equilibrium constants Kp and Kc should be lowercase.
Page 1033, Fi0 Entering molar flow of species i (mol/s) should read Fi0 Entering molar flow rate of species i (mol/s).
Page 1034, k Specific reaction rate should read k Specific reaction rate constant.
Page 1034, SD/U Selectivity parameter (instantaneous selectivity) (Chapter 6) should read SD/U Instantaneous selectivity (Chapter 6).
Page 1034, SD/U Overall selectivity of D to U should read ![]() D/U Overall selectivity of D to U.
D/U Overall selectivity of D to U.