Chapter 4
Example 4.2: Home Problem
The Laboratory Experiment
Here is a short movie about the use of the CSTR in the Senior Chemical Engineering Lab at the University of Michigan. You will need Quicktime to view these movies.
This Powerpoint Presentation details the reaction being carried out in the movie.
The hydrolysis of acetic anhydride is to be carried out at 25°C.

The water and acetic anhydride are mixed immediately before entering the reactor where the entering steam is 7.8 wt % acetic anhydride (1M) and 92.2 wt % water (51.2M). The volumetric feed rate of liquid in 0.0033 dm3/s. A one minute video clip of this experiment is given on the CDROM.
Additional Information
k'=1.97x10-4 dm3/mol s @ 25°C with E = 12,000 cal/mol
(a) What conversion can be achieved in a 1 dm3 CSTR at 25°C?
(b) What conversion can be achieved in a0.311 dm3 PFR?
(c) What time is required to achieve 80% conversion in a batch reactor?
The 460 Experiment Hydrolysis of Acetic Anhydride (AH)



|
|
A |
|
|
|
|
|
B |
|
|
|
|
|
C |
0 |
|
|





1. Mole Balance

Rearranging

2. Rate Law

3. Stoichiometry liquid v = v0






4. Combine


5. Evaluate
The maximum available flow rate in the experiment is 

V = 1 dm3
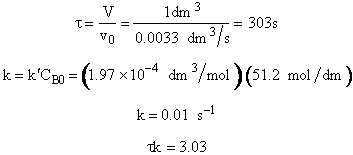

A.2. CSTRs in Series

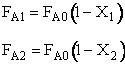

Balance on Second Reactor



1. Mole Balance/Design Equation

2. Rate Law

3. Stoichiometry


4. Combine


V = 0 X = 0


5. Evaluate


(c) Batch Reactor
1. Mole Balance/Design Equation

2. Rate Law

3. Stoichiometry
Liquid 

4. Combine



5. Evaluate











