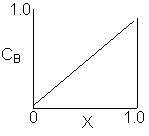
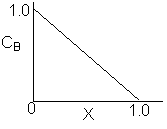
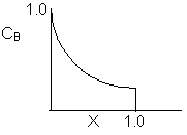
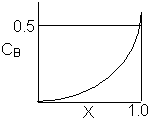
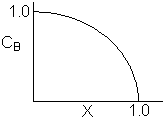
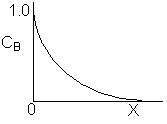
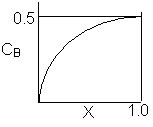
Match the following concentration- conversion curves (a-g) with the corresponding reaction and reaction conditions for a flow reactor at constant temperature and pressure.
| (a) | 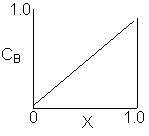 |
(b) | 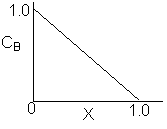 |
(c) | 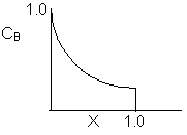 |
| (d) | 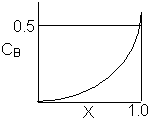 |
(e) | 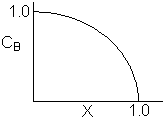 |
(f) | 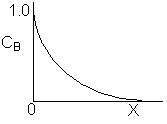 |
| (g) | 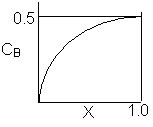 |
1) Gas phase, Pure A, CA0=1
![]()
2) Gas phase, CA0=CB0=1
![]()
3) Liquid phase, Pure A, CA0=1
![]()
4) Gas phase, Stoichiometric Feed, CA0=1/2
![]()
5) Gas phase, CA0=CB0=0.5
![]()
|
|
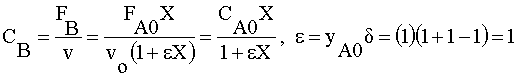 | ||
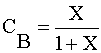 |
Ans(g) | |
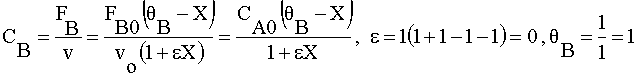 | ||
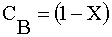 |
Ans(b) | |
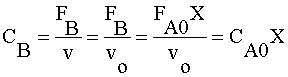 | |
Ans(a) |
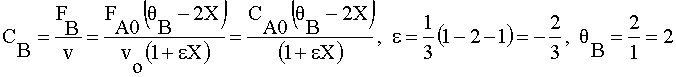 | ||
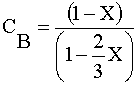 |
Ans(e) | |
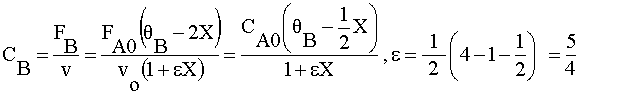 | ||
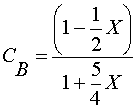 |
Ans(c) | |
Match the following concentration- conversion curves (a-g) with the corresponding reaction and reaction conditions for a flow reactor at constant temperature and pressure.
1) Gas phase, Pure A, CA0=1
2) Gas phase, CA0=CB0=1
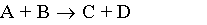
3) Liquid phase, Pure A, CA0=1
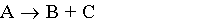
4) Gas phase, Stoichiometry Feed, CA0=1/2
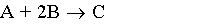
5) Gas phase, CA0=CB0=0.5
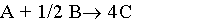
| (a) | 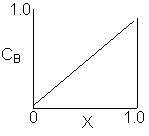 |
(b) | 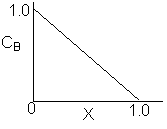 |
(c) | 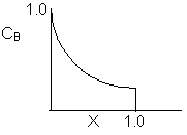 |
| (d) | 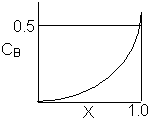 |
(e) | 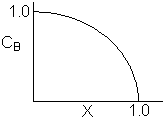 |
(f) | 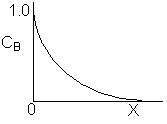 |
| (g) | 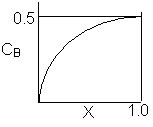 |
| 1) | 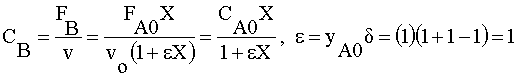 | |
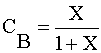 |
Ans(g) | |
| 2) | 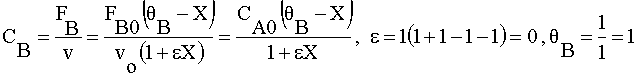 | |
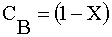 |
Ans(b) | |
| 3) | 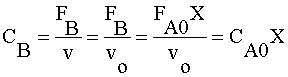 | |
Ans(a) | ||
| 4) | 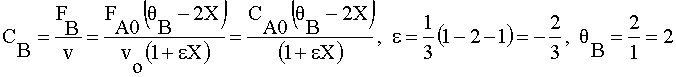 | |
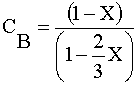 |
Ans(e) | |
| 5) | 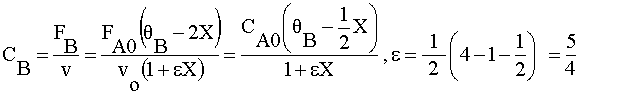 | |
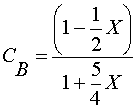 |
Ans(c) | |