The equations describing diffusion and reaction in porous catalysts also can be used to derive rates of tissue growth. One important area of tissue growth is in cartilage tissue in joints such as the knee. Over 200,000 patients per year that receive knee joint replacements. Alternative strategies include the growth of cartilage to repair the damaged knee.3
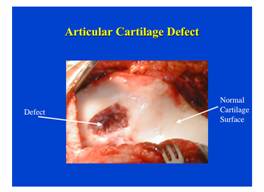
One approach currently being researched by Professor Kristi Anseth at the University of Colorado, is to deliver cartilage forming cells in a hydro gel to the damaged area such as the one shown in Figure E12-2.1.

Figure E12-2.1 Damaged cartilage. (Figure courtesy of Newsweek, September 3, 2001.)
Here the patient’s own Cells are obtained from a biopsy and embedded in a hydro gel, which is a cross-linked polymer network that is swollen in water. In order for these cells to grow in place, once embedded, it is critical that the tissue engineer tune the respiration rate of the gel to match the rate at which the cells secret extracellular matrix molecules (e.g., collagen). Because there is no blood flow through the cartilage, oxygen transport to the cartilage cells are primarily by diffusion. Consequently, the design must be that the gel can maintain the necessary rates of diffusion of nutrients (e.g., O2) into the hydro gel. These rates of exchange in the gel depend on the geometry and the thickness of the gel. To illustrate the application of chemical reaction engineering principles to tissue engineering, we will examine the diffusion and consumption of one of the nutrients, oxygen.
Our examination of diffusion and reaction in catalyst pellets showed that in many cases the reactant concentration near the center of the particle was virtually zero. If this condition were to occur in a hydro gel, the cells at the center would die. Consequently, the gel thickness needs to be designed to allow rapid transport of oxygen.
Let’s consider the simple gel geometry shown in Figure E12-2.2.
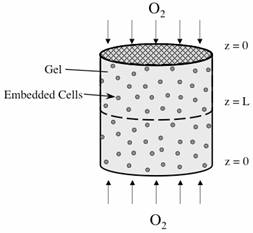
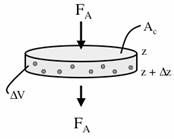
Figure E12-2.2 Schematic of cartilage cell system.
We want to find the gel thickness at which the minimum oxygen consumption rate is 10–13mol/cell/h, the cell density in the gel is 1010 cells/dm3, and the bulk concentration of oxygen (z = 0) is 2 x 10–4mol/dm3, and the diffusion rate is 10–5cm2/s.
Solution |
|||
A mole balance on oxygen, A, in the volume DV = AcDz is |
|||
|
(E12-2.1) | ||
Dividing by Dz and taking the limit as Dz ® 0 gives |
|||
|
(E12-2.2) |
||
|
(E12-2.3) |
||
For dilute concentrations we neglect UCA and combine Equations (E12-2.2) and (E12-2.3) to obtain |
|||
|
(E12-2.4) |
||
If we assume the O2 consumption rate is zero order, then |
|||
|
(E12-2.5) |
||
Putting our equation in dimensionless form using |
|||
|
(E12-2.6) |
||
Recognizing the second term is just the ratio of a reaction rate to a diffusion rate for a zero order reaction, we call this ratio the Thiele modulus, f0. We divide and multiply by two to facilitate the integration: |
|||
|
(E12-2.7) |
||
|
(E12-2.8) |
||
The boundary conditions are |
|||
At l = 0 y = 1 CA = CA0 |
(E12-2.9) |
||
At l = 1 |
(E12-2.10) |
||
Recall that at the midplane (z = L, l = 1) we have symmetry so that there is no diffusion across the midplane so the gradient is zero at l = 1. |
|||
Integrating Equation (E12-2.8) once yields |
|||
(E12-2.11) |
|||
Using the symmetry condition that there is no gradient across the midplane, Equation (E12-2.10), gives K1 = – 2f0: |
|||
|
(E12-2.12) |
||
Integrating a second time gives |
|||
| < align="center"> |
|||
Using the boundary condition y = 1 at l = 0, we find K2 = 1. The dimensionless concentration profile is |
|||
|
(E12-2.13) |
||
Note: The dimensionless concentration profile given by Equation (E12-2.13) is only valid for values of the Thiele modulus less than or equal to 1. This restriction can be easily seen if we set f0 = 10 and then calculate y at l = 0.1 to find y = –0.9, which is a negative concentration!! This condition is explored further in Problem P12-10B. |
|||
Parameter Evaluation |
|||
Evaluating the zero-order rate constant, k, yields |
|||
|
|||
and then the ratio |
|||
|
(E12-2.14) |
||
The Thiele module is |
|||
|
(E12-2.15) |
||
(a) Consider the gel to be completely effective such that the concentration of oxygen is reduced to zero by the time it reaches the center of the gel. That is, if y = 0 at l = 1, we solve Equation (E12-2.13) to find that f0 = 1 |
|||
|
(E12-2.16) |
||
Solving for the gel half thickness L yields |
|||
|
|||
Let’s critique this answer. When the oxygen concentration is zero at the center, there is no driving force for oxygen to diffuse across the cell membrane; hence, it is most likely the cells will die. |
|||
(b) Now consider the minimum concentration at the center to be 0.1 mol/dm3 which is one half that at the surface (i.e., y = 0.5 at l = 1.0). Then Equation (E12-2.13) gives |
|||
|
(E12-2.17) |
||
Solving equation (E12-2.17) for L |
|||
|
|||
Consequently, we see that the thickness of the cartilage gel (2L) is the order of 1 mm. |
|||
(c) One can consider other perturbations to the preceding analysis by considering the reaction kinetics to follow a first-order rate law, –rA = kACA, or Michaelis-Menten kinetics, |
|||
|
(E12-2.18) |
||
The author notes the similarities to this problem with wax build-up in subsea pipeline gel.4 Here as the paraffin diffuses into the gel to form and grow wax particles, these particles cause paraffin molecules to take a longer diffusion path, and as a consequence the diffusivity is reduced. An analogous diffusion pathway for oxygen in the hydro gel containing collagen is shown for in Figure E12-2.3. |
|||
|
|||
Figure E12-2.3. Diffusion of O2 around collagen. |
|||
|
(E12-2.19) |
||
where a and Fw are predetermined parameters that account for diffusion around particles. Specifically, for collagen, a is the aspect ratio of the collagen particle and Fw is weight fraction of “solid” collagen obstructing the diffusion. A similar modification could be made for cartilage growth. These situations are left as an exercise in the end-of-the chapter problems, e.g., 12-2(b).
|
3 http://www.genzymebiosurgery.com/prod/cartilage/gzbx_p_pt_cartilage.asp.
4 P. Singh, R. Venkatesan, N. Nagarajan and H. S. Fogler, AIChE J., 46, 1054 (2000).