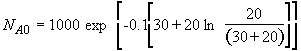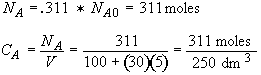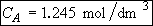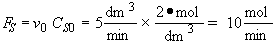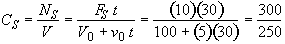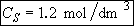
A → B Acid Catalyzed
The irreversible liquid phase acid catalyzed isomerization reaction
is carried out isothermally in a semibatch reactor.
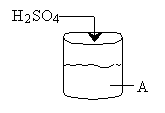
A 2 molar solution of H2SO4 is fed at a constant rate of 5 dm3/min to a reactor that initially contains no sulfuric acid. The initial volume of pure A solution in the reactor is 100 dm3. The concentration of pure A is 10 mol/dm3. The reaction is first order in A and first order in catalyst concentration and the specific reaction rate is 0.05 dm3/mol min–1. The catalyst, of course, is not consumed during the reaction.
a) Write a complete POLYMATH program (line by line in POLYMATH notation) to determine both the number of moles of A and of H2SO4 in the reactor and the concentration of A and of H2SO4 as a function of time. Use the mole balance expressed in terms of NA.
b) Obtain an analytical solution for the number of moles of A, NA and the concentration of A, CA, as a function of time. What is the concentration of A and of H2SO4 after 30 minutes?
Additional Information
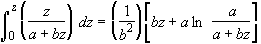
Solution:
(a) POLYMATH Solution
Mole Balance
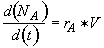
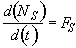
Rate Law and Stoichiometry
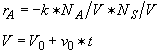
Parameters
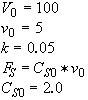
Polymath will combine
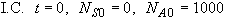
(b) Analytical Solution
A B
B
Mole Balance
![]() (SS-1)
(SS-1)
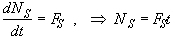 (SS-2)
(SS-2)
Rate Law
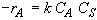 (SS-3)
(SS-3)
Stoichiometry
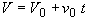 (SS-4)
(SS-4)
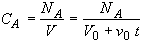 (SS-5)
(SS-5)
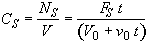 (SS-6)
(SS-6)
Combine
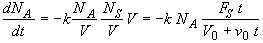 (SS-7)
(SS-7)
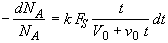 (SS-8)
(SS-8)
Dividing by v0 and recalling t0 = V0/v0
![]() (SS-9)
(SS-9)
Using the integral
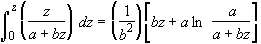 (SS-10)
(SS-10)
with
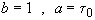
t = 0 NA = NA0
we obtain
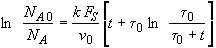 (SS-11)
(SS-11)
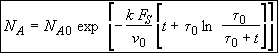 (SS-12)
(SS-12)
Note! If one does not divide by v0 in Equation (SS-8), then
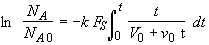 (SS-13)
(SS-13)
Here b = v0, a = V0
The integral becomes
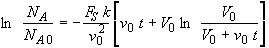 (SS-14)
(SS-14)
Factoring out a v0 from the term in brackets
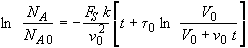 (SS-15)
(SS-15)
we see we get the same result as Equation (SS-11).
Evaluate
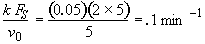
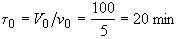
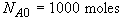
At t = 30 We substitute into Equation (SS-12)