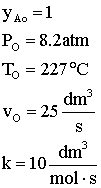
Problem:
The elementary irreversible gas phase reaction 2A -> B is carried out isothermally with no pressure drop in a PFR.
Given:
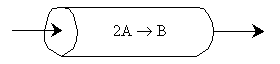
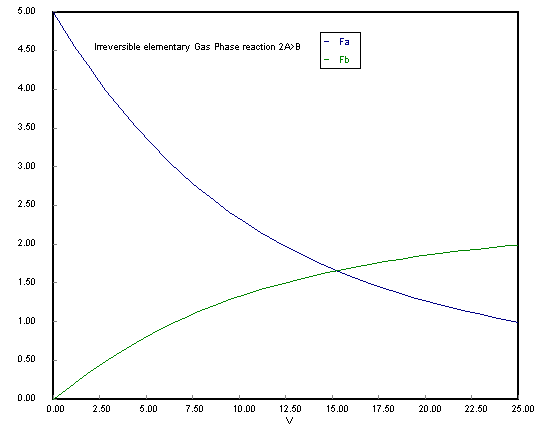
Use measures other than conversion to plot molar flow rates of A and B down the reactor.
Hint 1: What are the mole balances on A and B and the rate law?
Hint 2: What are the concentrations of A and B?
Hint 3: What is the rate of formation of B?
Hint 4: What are the combined mole balance, rate law and stoichiometry?
Hint 5: What is the polymath program?
Mole Balances
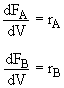
Rate Law
![]()
Stoichiometry
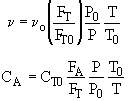
For Isothermal and No DP
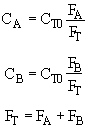
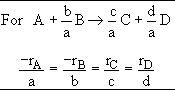
For 2A->B
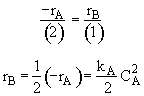
Combine
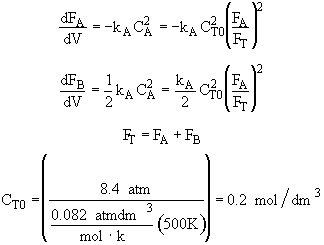
[Note: To=227C=500K]![]()
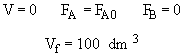
Use Polymath to solve
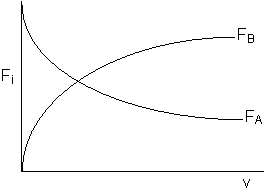
One can back calculate X
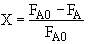
See the Polymath Solution Below
|
POLYMATH 5.0 Results 01-19-2001 Calculated values of the DEQ variables
ODE Report (RKF45) Differential equations as entered by the user [1] d(Fa)/d(V) = -ka*Cto^2*(Fa/Ft)^2 [2] d(Fb)/d(V) = 0.5*ka*Cto^2*(Fa/Ft)^2 Explicit equations as entered by the user [1] Cto = 0.2 [2] ka = 10 [3] Ft = Fa+Fb [4] x = (5-Fa)/5
|
Mole Balances
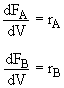
Rate Law
![]()
Stoichiometry
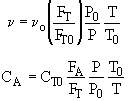
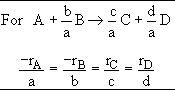
For 2A->B
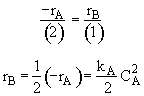
For Isothermal and No DP
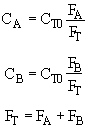
Combine
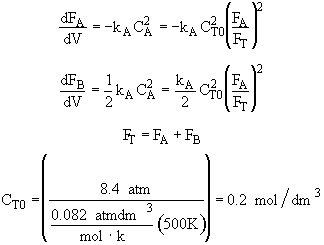
[Note: To=227C=500K]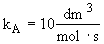
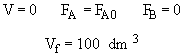
Use Polymath to solve

One can back calculate X
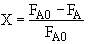
See the Polymath Solution Below
|
POLYMATH 5.0 Results 01-19-2001 Calculated values of the DEQ variables
ODE Report (RKF45) Differential equations as entered by the user [1] d(Fa)/d(V) = -ka*Cto^2*(Fa/Ft)^2 [2] d(Fb)/d(V) = 0.5*ka*Cto^2*(Fa/Ft)^2 Explicit equations as entered by the user [1] Cto = 0.2 [2] ka = 10 [3] Ft = Fa+Fb [4] x = (5-Fa)/5
|
