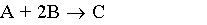
The zero order reaction
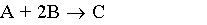
is carried out in a CSTR and in a PFR. For an entering volumetric flowrate of 10 dm3/s at a concentration of .5 mol/dm3, what is the space time for each reactor to achieve 90% conversion.
Additional Information: kA = 0.01 mol/dm3•s
Hint 2: CSTR combined mole balance and rate law
MOLE BALANCE: |
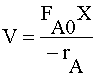 |
RATE LAW: |
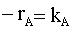 |
COMBINE: |
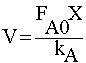 |
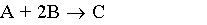
is carried out in a CSTR and in a PFR. For an entering volumetric flowrate of 10 dm3/s at a concentration of .5 mol/dm3, what is the space time for each reactor to achieve 90% conversion.
| MOLE BALANCE: | 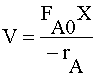 |
| RATE LAW: | 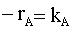 |
| STOICHIOMETRY: |
|
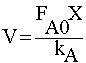 |
|
| COMBINE: | |
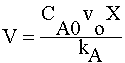 |
|
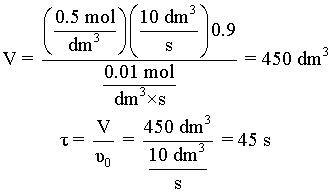 |
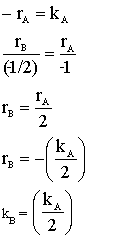
is carried out in a CSTR and in a PFR. For an entering volumetric flowrate of 10 dm3/s at a concentration of .5 mol/dm3, what is the space time for each reactor to achieve 90% conversion.
| MOLE BALANCE: |
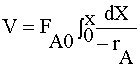 |
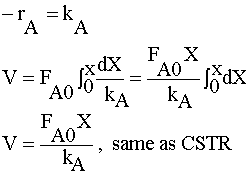
|