The surface reaction is rate limiting. We need to further discuss setting (rAD/kA)~0 to arrive at CC.S=KIPICv. We are going to do this by first using the total number of sites, CT [i.e. CT=Cv+CA.S+CB.S], and then use the fractional surface coverage [1=fv+fA.S+fB.S].
![]()
![]()
![]()
For steady state operation we have:
![]()
where:
![]()
![]()
NOTE: Strictly speaking, We really cannot compare the magnitudes of kA, kS, and kD directly, because kA has different units than kS and kD. Consequently, we must compare the product (kAPC) with kS and kD to determine which reaction step may be limiting. If the surface reaction is limiting, we say kAPC and kD are very large with respect to kS:
Strictly speaking, We only take ratios of quantities that have the same units.
![]()
If![]() and
and![]() are the
fraction of free sites and the fraction of covered sites, respectively, and
are the
fraction of free sites and the fraction of covered sites, respectively, and![]() is the
gas phase mole fraction of species A, then:
is the
gas phase mole fraction of species A, then:
![]()
![]()
![]()
and
![]()
For surface reaction control:
![]()
![]()
which is identical to the expression derived in the text, assuming that:
![]()
Developing the Rate Law
Now let's consider developing the rate law on the basis of fractional suface coverage, i.e. (rAD/kA)~0 and (kADB/kB)~0.

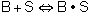
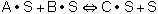
Assume that the surface reaction is limiting, then:
Table 10-4 Irreversible Surface-Reaction-Limited Rate Laws.
NOTE: This is the same rate law we would get by comparing the k's directly. Return to Chapter