eResearch Release 2.2
Scheduled release date: Monday, November 16, 2009
Release 2.2 system outage information:
5 pm Friday, November 13 through 6 am, Monday, November 16
- New design for entering Benefits and Risk in Section 6
- Section 14-1 Clinical Research Billing Unit changes
- Sections 7, 15, 16 Drug and Device updates
- Reviewer functionality improvements
For a listing of all changes included in the release, refer to the release notes.
New design for entering Benefits and Risk in Section 6
Section 6 has been updated to allow for the entry of multiple risk levels in one application.
Study teams will respond to 6.4 (select No if there is one risk level for the study, or Yes if there is more than one risk level for the study):

Click Add to enter the risk level information on the detail page.
If there is one risk revel (response to 6.4 is No), the detail page will begin with question 6.6 (6.5.1 and 6.5.2 will not display).
If there are multiple risks levels (response to 6.4 is Yes), the detail page will begin with question 6.5.1.
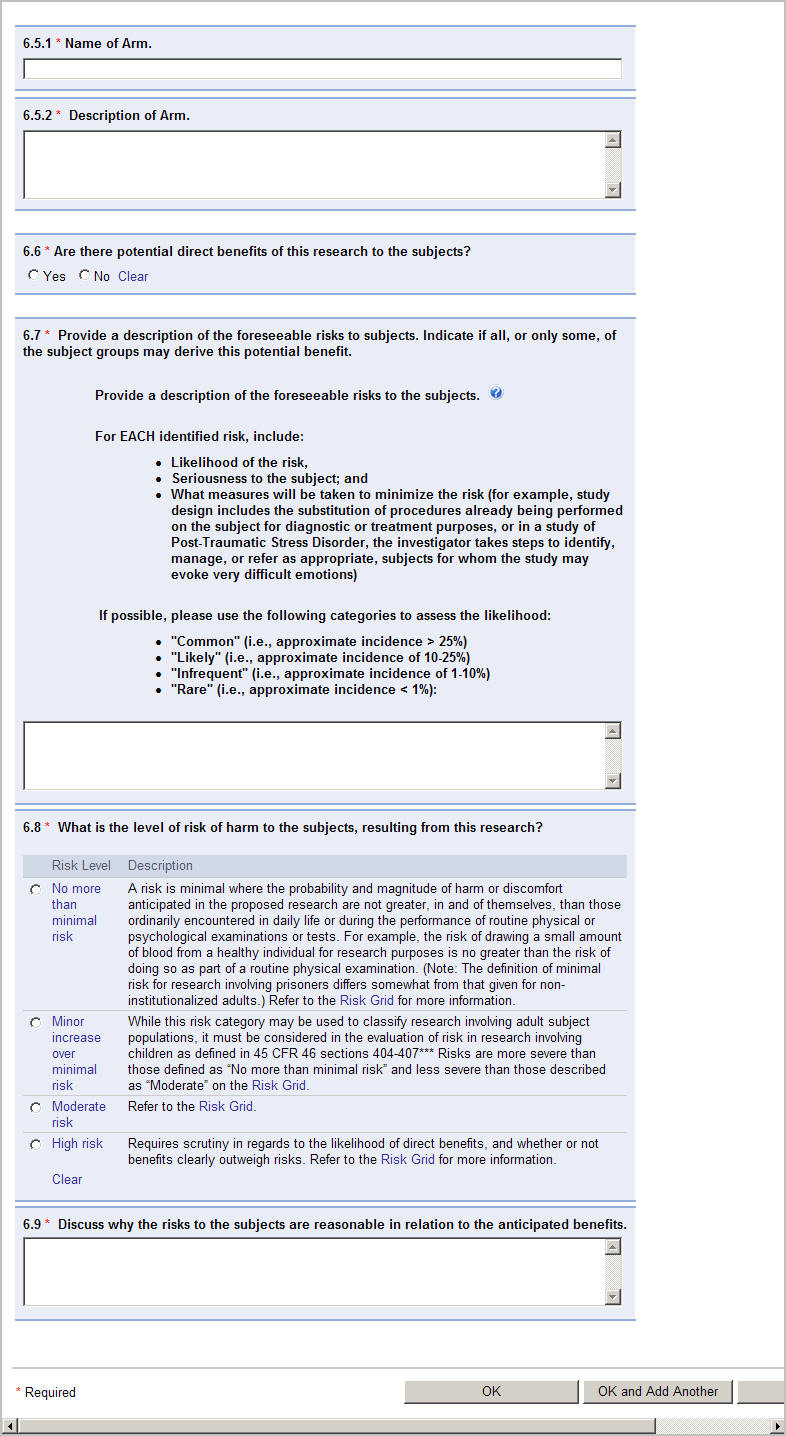
Complete the questions and click OK or OK and Add Another to save the information.

The information will save in question 6.5. Click Edit to return to the detail page for that risk level.
NOTES:
- The default name for all converted risk level information is the study ID.
- Study teams change existing risk levels in an SCR (Continuing Review). To add a risk level, study teams need to submit an AME (amendment).
- Core Staff: if an AME and SCR are both in progress, the submission saved last will be risk level saved to the parent application. Please confirm that the risk levels match.
Section 14-1 Clinical Research Billing Unit Changes
Questions 14-1.1 - 14-1.4 have been combined into one question. Study teams who previously responded to 14-1.1 - 14-1.4 will now need to respond to the new question 14-1.1.
Sections 7, 15, 16 Drug and Device Updates
Changes to sections 7, 15, and 16 for Drugs and Devices
New question: 7-2.1.1 Are the following statements ALL true regarding ALL the device(s) used in Research
- Legally marketed in the United States
- Used "on-label" (i.e., consistent with the FDA-approved labeling or manufacturer instructions)
- Have no post-manufacturing modifications?
Note: Any devices being used in the research procedures should be described in either the scientific protocol document (question 5.1.1) or in Research Design-Methodology (question 5-1.5). Yes/No
If No, Section 16 will be required
If yes, Section 16 is not required (there are two additional questions in Section 7)
Sections 15 and 16 have been updated
Reviewer Functionality Improvements
Inbox routing has been updated so items that reviewers need to take action on will appear in the committee member workspace Inbox (some items previously displayed on the In Progress tab)
The committee member workspace will display the agenda date and committee for full committee submissions
Staff review checklists will update when the study team makes significant changes to the submission
Routing for contingencies under review has been updated