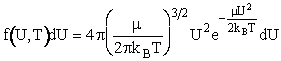

where f(ε,T) de is the fraction of molecules with kinetic energies between ε and (ε+dε). We could further multiply and divide by kBT
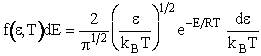
Recalling ![]()
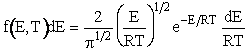
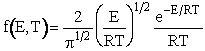
By letting ![]() , we could have put the distribution in dimensionless form
, we could have put the distribution in dimensionless form
![]()
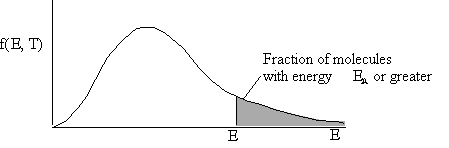
f(X,T) dX is the fraction of molecules that have energy
ratios between ![]() and
and
![]()
Return to Collision Theory