First, we will list the known values:
AgClO4 + CH3I![]() CH3ClO4 + AgI
CH3ClO4 + AgI
A + B![]() C + D
Batch Process
C + D
Batch Process
V = 30 dm3
CAo = 0.5 mol/dm3
CBo = 0.7 mol/dm3
rB = -kCBCA3/2 at T = 298 K
k = 0.00042 (dm3/mol)3/2/s
Final X = 0.98
Mole Balance
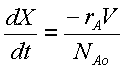
Rate Law
rA = rB = -kCBCA3/2
Stoichiometry
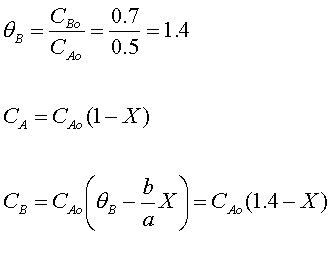
Combine
Combining the above equations, we arrive at:
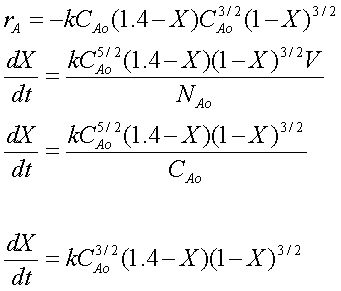
Evaluate
The final equation, with all values entered, is:
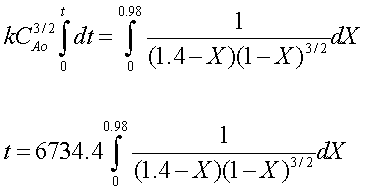
This equation can only be solved numerically, unless you really enjoy integration. The Polymath simultaneous differential equation solver may be used to solve the problem quickly and easily. The Polymath equations used should look like this:
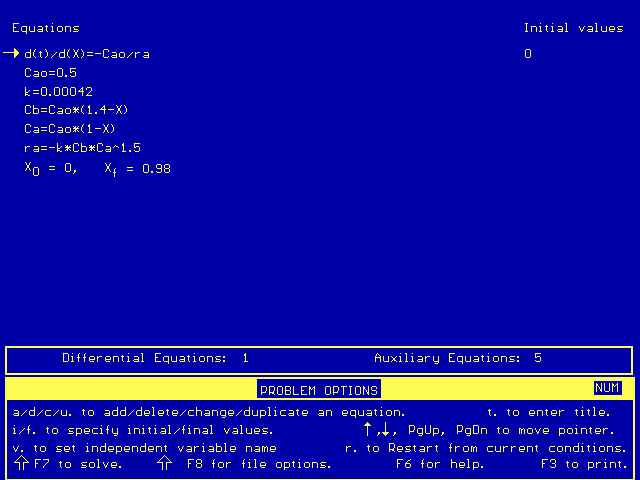
After Polymath solves the equations, it gives a chart of initial, maximum, minimum, and final values:
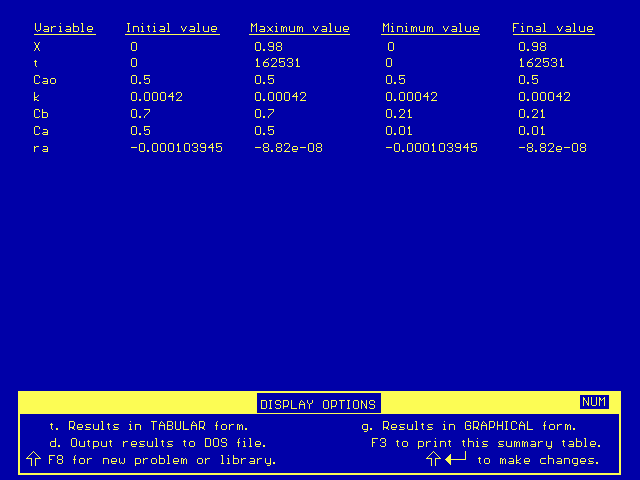
From these results, we can see that the final time (and therefore the time to reach 98% conversion) is
t = 162531 s = 45.15 hr