Additional Homework Problems
CDP6-QB
The gas-phase reaction A+2B → 2D is
to be carried out in an isothermal plug-now reactor at 5.0 atm. The mole fractions
of the feed streams are A = 0.20, B = 0.50, and inerts = 0.30.
(a) What is the steady-state volumetric flow rate at any
point in the reactor if the pressure drop due to fluid friction can be ignored? [Ans.:
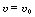 (1 - 0.2X).]
(1 - 0.2X).]
(b) What are the expressions for the concentrations of A, B, and D as a function
of conversion at any point along the reactor?
(c) What is the feed concentration (units: mol/ dm 3
) of A if the feed temperature is 55°C?
(d) Determine how large the plug-flow reactor must be to achieve a conversion
(based on A) of 0.70 if the temperature in the reactor is uniform (55°C), the
volumetric feed rate is 50 dm 3 /min,
and the rate law at 55°C is
-r = 2.5 C A(1/2) C B kmol/m 3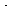 min
min
(Ans.: V = 50.21 dm 3.)
(e) Plot the concentrations, volumetric flow rate, and conversion
as a function of reactor length. The reactor diameter is 7.6 cm.
(f) How large would a CSTR have to be to take the effluent from the PF reactor
in part (d) and achieve a conversion of 0.85 (based on the feed of A to the plug-flow
reactor) if the temperature of the CSTR is 55°C?
(g) How many 1-in.-diameter pipe tubes, 20 ft in length, packed with a catalyst,
are necessary to achieve 95% conversion of A starting with the original stream? Plot
the pressure and conversion as a function of reactor length. The particles are 0.5
mm in diameter and the bed porosity is 45%.
(h) Calculate the PFR size to achieve 70% of the equilibrium conversion and
the CSTR size necessary to raise the conversion of the PFR effluent to 85% of the
equilibrium conversion if their temperatures were uniform at 100°C. The activation
energy for the reaction is 30 kJ/mol, and the reaction is reversible with an equilibrium
constant at 100°C of 10 (m 3 /kmol) 1/2
. (Ans: V PFR = 8.56 dm
3 , V CSTR
= 6.45 dm 3 )
[2nd Ed. P4-8]