Additional Homework Problems
CDGA 6-2A
(CSTR train) The elementary liquid-phase reaction
A + B 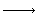 C
C
is to be carried out in a CSTR with three impellers. The mixing patterns in the CSTR are such that it is modeled as three equal-sized CSTRs in series. Species A and B are fed in separate lines to the CSTR, which is initially filled with inert material. each CSTR is 200 dm3 and the volumetric flow to the first reactor is 10 dm3/min of A and 10 dm3/min of B.
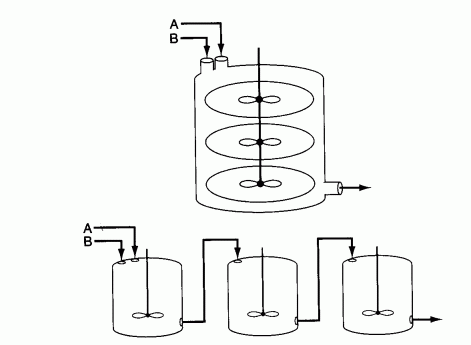
- What is the steady-state conversion of A?
- Determine the time necessary to reach steady state (i.e., when CA exiting the third reactor is 99% of the steady-state value).
- Plot the concentration of A exiting each tank as a function of time.
- Suppose that the feed for species B is split so that half is fed to the first tank and half to the second tank. Repeat parts (a), (b), and (c).
- Vary the system parameters, v0, V, k and so on, to determine their effects on startup. Write a paragraph describing the trends you found which includes a discussion of the parameter that most effects the results.
Additional Information
CA0 = CB0 = 2.0 mol/dm3
k = 0.025 dm3/mol/min
[3rd Ed. P4-29]
![]() C
C
