Additional Homework Problems
CDP6-LB
The reversible isomerization
A 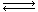 B
B
is to be carried out in a membrane reactor (IMRCF). Owing to the configuration of species B, it is able to diffuse out the walls of the membrane, while A cannot.
- What is the equilibrium conversion assuming that B does not diffuse out of the reactor walls?
- Plot the conversion profiles to compare a 100 dm3 conventional PFR with a 100 dm3 membrane reactor. What statements or generalizations can you make? What parameters have the greatest effect on the shape of the exit conversion plots in part (A)?
- Plot the conversion and species concentrations and the molar flow rates down the length of the reactor.
- Vary some of the parameters and write a paragraph describing your results.
- Discuss how your curves would change if the temperature were increased significantly or decreased significantly for an exothermic reaction and for an endothermic reaction.
Additional Information
Specific reaction rate = 0.05 s-1
Transport coefficient kC = 0.3 s-1
Equilibrium constant Ke = 0.5
Entering volumetric flow rate v0 = 10 dm3/s
CA0 = 0.2 mol/dm3
[3rd Ed. P4-30]
![]() B
B