A liquid organic substance, A, contains 0.1 mol % of an impurity, B, which can be hydrogenated to A:
B+H2 → A
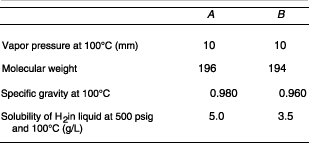
The material is purified by hydrogenation as a liquid in a continuous well-mixed reactor at 100°C. The feed rate of the liquid is constant at 730 lb/h. The reactor holds 50 gal of liquid, at 500 psig, and the amount of B in the product levels out at 0.001 mol %. What will be the concentration of B in the product if the hydrogen pressure is held at 300 psig? Assume that the reaction behaves as though it were first order with respect to both B and H 2 , that is, in batch,
![]()

Assume perfect gas laws and Henry's law. Also assume the following
properties:
[2nd Ed. P4-15]