The following data were obtained at 100°C in a batch reactor using sulfided CoMo as a catalyst at a concentration of 20 g/dm 3 .
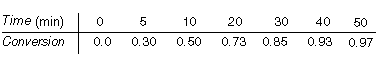
Also note that the rate at 110° C is approximately four times the rate at 80°C. Verify that the reaction is pseudo-first order in 5,6-benzoquinoline and determine the specific reaction rate.
It has been learned that the specific reaction rate is directly proportional to the catalyst concentration (i.e., first order). It is proposed to double the catalyst concentration and drop the temperature to 90°C. Plot the conversion expected under these conditions as a function of time and compare with the data above.
[2nd Ed. P4-7]