Additional Homework Problems
The elementary liquid phase reaction
A + B 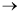 C
C
is carried out in a 500 dm3 reactor. The entering concentrations of streams A and B are both 2-molar and the specific reaction rate is 0.01 dm3/(mol*min).
- Calculate the time to reach 90%conversion if the reactor is a batch reactor filled to the brim.
Assuming a stoichiometric feed(10 mol A/min) to a continuous flow reactor, calculate the reactor volume and space-time to achieve 90% conversion if the reactor is
- a CSTR (Ans: V = 22,500dm3)
- a PFR (Ans: V = 2,250 dm3)
- Redo (a) through (c) assuming the reaction is first-order in B and zero-order in A with k = 0.01 /min.
- Assume the reaction is reversible with Ke = 2 dm3/mol. Calculate the equilibrium conversion and the CSTR and PFR volumes necessary to achieve 98% of the equilibrium conversion.
![]() C
C