Solution
Recalling Example 7-1, the combined mole balance and rate law for a constant-volume batch reactor can be expressed in the form![]()
![]()
![]()
Assuming a zero-order reaction
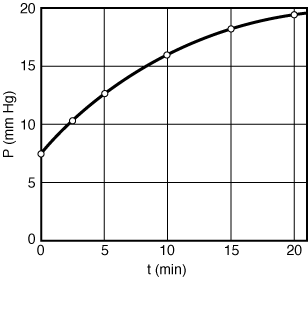
Figure CDE7-1-1
![]()
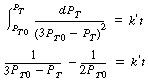
![]()
![]()
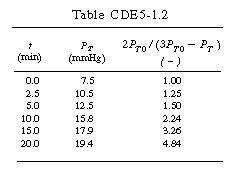
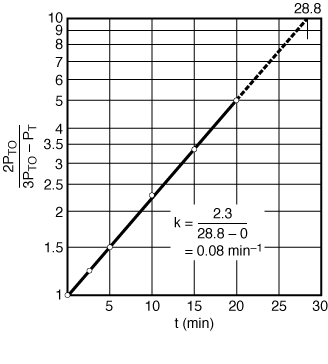
Figure CDE7-1.3
Use the integral method to determine the reaction order for the di-tert-butyl peroxide decomposition described in Example 7-1.SolutionRecalling Example 7-1, the combined mole balance and rate law for a constant-volume batch reactor can be expressed in the form |
||
|
(E7-1.5) | |
| As a first guess we might try zero order, |
||
|
(CDE7-1.1) | |
| Integrating gives us | ||
|
|
||
Assuming a zero-order reaction |
If this is the correct order, a plot of PTversus t should be linear. After using the data in Table CDE7-1.1 to obtain Figure CDE7-1.1, we see that PT is not a linear function of t. Consequently, we conclude that the reaction is not zero-order. | |

Figure CDE7-1-1 |
||
| Next we try second order, |
||
|
||
| Integrating yields | ||
|
||
| Assuming a second-order reaction | If the reaction is second order, a plot of |
|
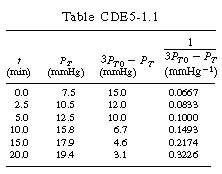
| After forming Table CDE7-1.1, a plot of |
||
|
||
 |
||
| Finally, we try first order (i.e. |
||
|
||
| Integrating with limits, |
||
|
||
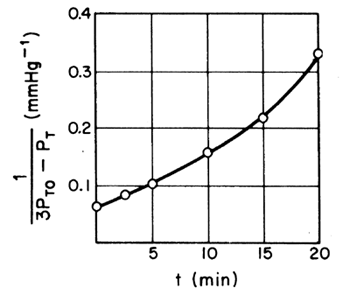
| If the reaction is first order, a plot of |
||
|
||
 Figure CDE7-1.3 |