The oxidation of propene (P) to acrolein (A) was carried out over a Mo-Pr-Bi catalyst [Ind. Eng. Chem. Res., 26, 1419 (1987)].
CH3CH=CH2+O2![]() CH2=CHCHO+H2O
CH2=CHCHO+H2O
It has been proposed to correlate the data using the power law model for the rate law [cf. Equation (5-2)].
racrolein![]()
The reaction was carried out in a differential reactor with 0.5 g of catalyst at 623 K. From the data below, determine the reaction orders with respect to propene![]() and oxygen
and oxygen![]() and the specific reaction rate, k.
and the specific reaction rate, k.
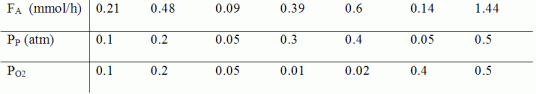
where
FA=exiting molar flow rate of acrolein, mmol/h
PP= entering partial pressure of propene, atm
PO2= entering partial pressure of oxygen, atm