Mixtures of hydrocarbons (e.g., petroleum feedstocks) that undergo cracking reactions or hydroemethylation can sometimes be lumped as just one reactant or as two or more reactants. In many cases it is difficult to distinguish lumping as a single reactant with second-order kinetics from lumping as two reactants each with first-order kinetics. [Ind. Eng. Chem. Process Des. Dev., 19, 197 (1980)]. To distinguish between these two cases, the initial reactant concentration must be varied in more than one run and conversions greater than 92% should be sought in taking the data.
From the batch reactor data below, determine whether first-order kinetics for lumping as two reactants A and B or second-order kinetics for lumping one reactant D best describes the system. Experimental conditions are such that one can neglect volume change.
![]()
![]()
![]()
![]()
![]()
Only the total concentration of the lumped reactant, C(t), can be monitored as a function of time. For two-parameter lumping, estimates of the initial concentrations are CA0=0.008 kmol/m3 and CB0=0.006 kmol/m3.
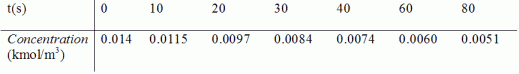
Where would you place additional data points?