To solve this problem, we must follow the usual algorithm for multiple reactions with catalysis.
Mole Balance
Since there are four components, there are four mole balances for this problem. They are:
IN - OUT + ACCUM = 0
voCAo - voCA +
rAW = 0
-voCB + rBW = 0
-voCC + rCW = 0
-voCD + rDW = 0
Remember, from the problem statement, that the volume change with reaction can be neglected since the reactions are carried out in excess air. Therefore, v = vo.
Rate Laws
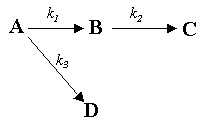
Next, the rate laws must be formed, using the reaction sequence diagram and noting that Reaction 1 is second-order while Reactions 2 and 3 are first-order.

The rate laws become:
rA = -k1CA2 -
k3CA
rB = k1CA2 -
k2CB
rC = k2CB
rD = k3CA
Polymath Solution
This set of equations, along with the reaction parameters in the problem statement, may be entered into Polymath to find the exiting concentrations. The equations entered in polymath are as follows:
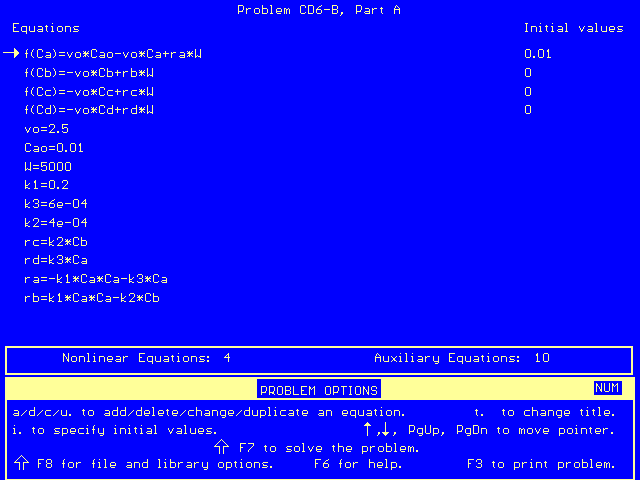
Polymath then solves the equations and gives us an output file:
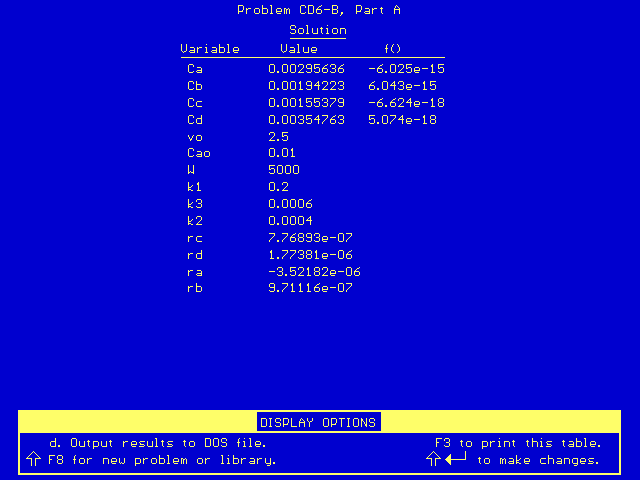
The final concentration values are listed in this results table. Therefore, the exit concentrations from a CSTR in this problem are:
CA = 2.96 x 10-3
mol/dm3
CB = 1.94 x 10-3 mol/dm3
CC = 1.55 x 10-3 mol/dm3
CD = 3.55 x 10-3 mol/dm3
Selectivity is a ratio of reaction rates. Therefore, the selectivity of B to C is the ratio of rB and rC, and the selectivity of B to D is the ratio of rB and rD.
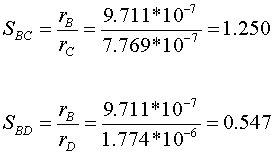
To solve this problem, we must set the up equations needed for a PBR with multiple reactions. The mole balances are:
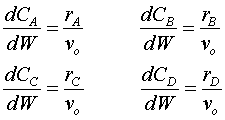
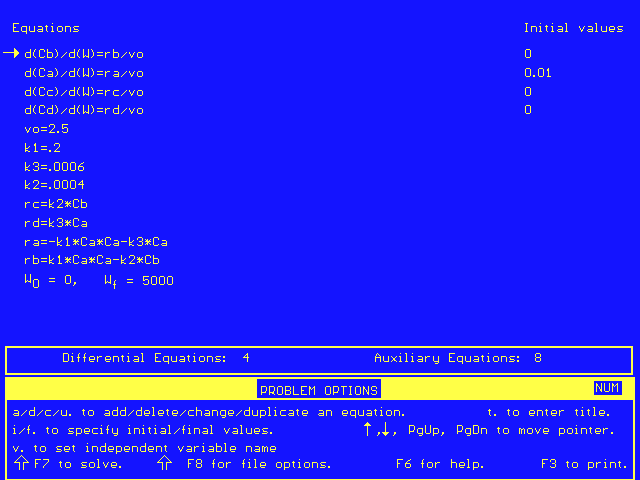
These, combined with the same rate laws as in Part A, give the following Polymath equations:
These equations result in the following plot of concentrations as a function of catalyst weight in the PBR.
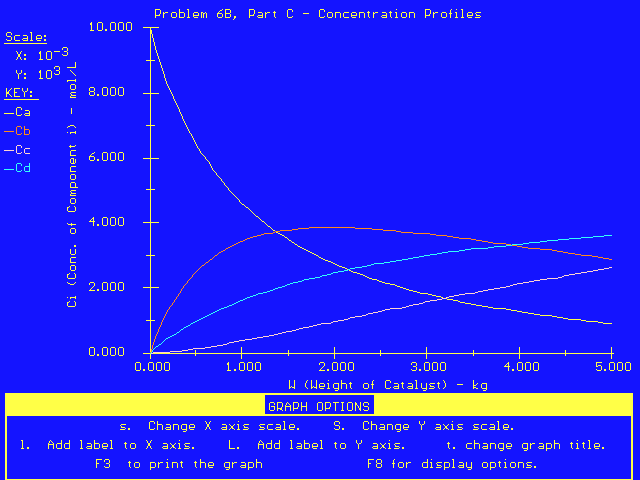
From this plot, we can see that the concentration of B is a maximum at a catalyst weight of approximately 2000 kg. To get the exact answer, we display CB as a function of W in a table of values:
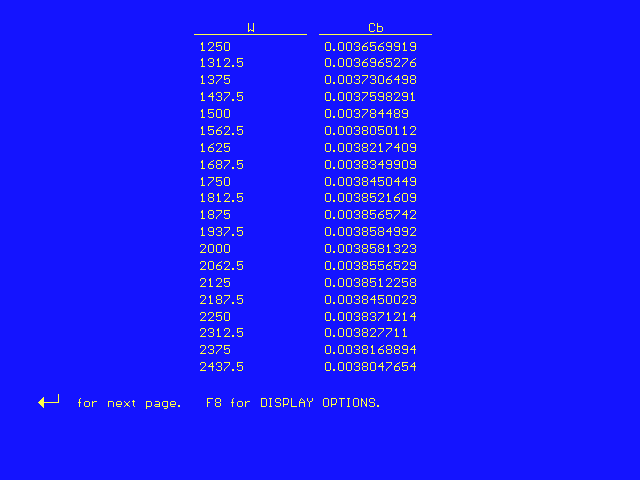
This table shows that the concentration of B is a maximum at a catalyst weight of 1937.5 kg.
To model these changes in CAo for the PFR, we just replace the original value of CA in the Polymath equations above. The new product distribution, with CAo as a larger value (0.5 mol/dm3) is:
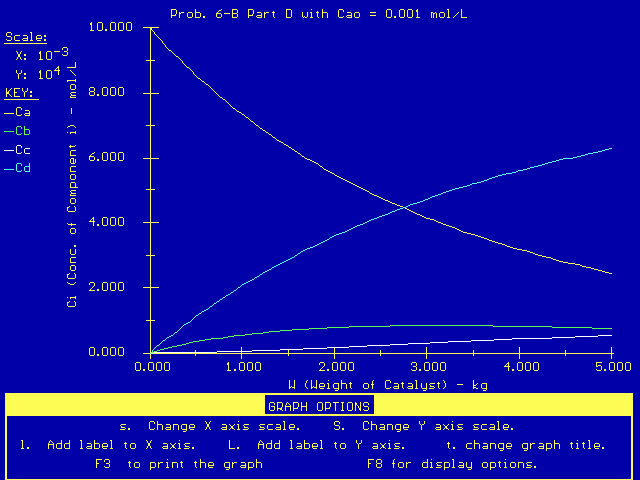
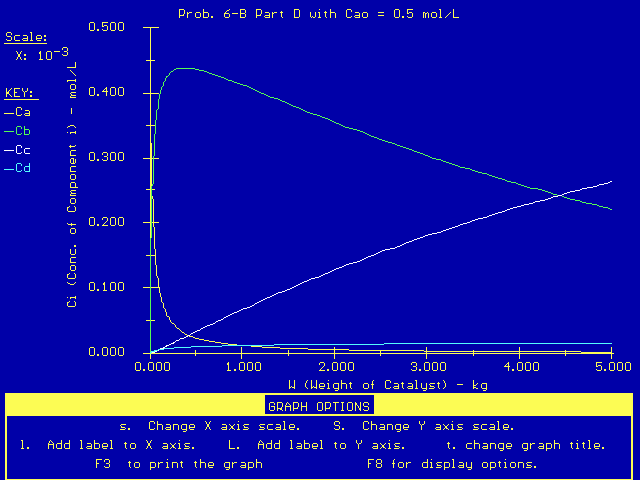 Also, if we change the entering concentration of CA to a very
low value, such as 0.001 mol/dm3, we get this product
distribution:
Also, if we change the entering concentration of CA to a very
low value, such as 0.001 mol/dm3, we get this product
distribution: